PDK1 (Phosphoinositide-dependent kinase-1) also known as PDPK1 (3-phosphoinositide dependent protein kinase-1) is a serine/threonine protein kinase, which functions as a master kinase to phosphorylate and activate a subgroup of the AGC family kinases such as PKB/Akt, PKC, S6K, SGK, and RSK, thus, playing a pivotal role in the PI3K/Akt signaling pathway. [show the full text]
PDK inhibiteurs (PDK Inhibitors)
| N° de cat. | Nom du produit | Informations | Citations dutilisation du produit | Validations de produit |
|---|---|---|---|---|
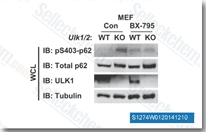
| S1274 | BX-795 | Le BX-795 est un inhibiteur puissant et spécifique de PDK1 avec une IC50 de 6 nM, 140 et 1600 fois plus sélectif pour PDK1 que pour PKA et PKC dans les essais sans cellules, respectivement. Parallèlement, par rapport à GSK3β, une sélectivité plus de 100 fois supérieure a été observée pour PDK1. Le BX-795 module l'autophagy via l'inhibition de ULK1. Le BX-795 est également un puissant inhibiteur de TBK1 qui bloque à la fois TBK1 et IKKε avec des valeurs d'IC50 de 6 nM et 41 nM, respectivement. |
|
|
| S0983 | JX06 | JX06 est un inhibiteur covalent sélectif de PDK1 dans les cellules. Ce composé inhibe PDK1, PDK2 et PDK3 de manière dose-dépendante avec des IC50 de respectivement 0,049 μM, 0,101 μM et 0,313 μM. |
|
|
| S8615 | Sodium Dichloroacetate (DCA) | Le DCA (Sodium Dichloroacetate), un inhibiteur spécifique de la pyruvate dehydrogenase kinase (PDK) avec des valeurs d'IC50 de 183 et 80 μM pour PDK2 et PDK4 respectivement, a démontré qu'il déréprime le cotransporteur Na+-K+-2Cl- et un axe mitochondrial de canal potassique. Le Sodium Dichloroacetate augmente la génération de reactive oxygen species (ROS), déclenche l'apoptose dans les cellules cancéreuses et inhibe la croissance tumorale. |
|
|
| S2949 | KPLH1130 | KPLH1130 est un inhibiteur spécifique de la pyruvate déshydrogénase kinase (PDK) qui bloque la polarisation des macrophages et atténue les réponses pro-inflammatoires. |
PDK1 protein is composed of two well-characterized functional domains: (1) the N-terminal serine/threonine kinase domain of the AGC family, and (2) the C-terminal Pleckstrin homology (PH) domain that interacts with high affinity with both PtdIns(3,4,5)P3 and PtdIns(3,4)P2 as well as other phosphoinositides such as PtdIns(4,5)P2. PDK1 is ubiquitously expressed and constitutively active in mammalian cells. In addition to being able to constitutively phosphorylate substrates, PDK1 also works in an inducible manner in response to specific stimuli. Without stimuli, the catalytic activity of PDK1 is kept under control by limiting its access to targets, whereas upon stimulation, the different PDK1 substrates are converted into forms that can be recognized, phosphorylated and activated by PDK1. PDK1 regulates as much as 23 growth-factor-stimulated AGC kinases, containing protein kinase B (PKB/Akt) isoforms, p70 ribosomal protein S6 kinase (S6K) isoforms, p90 ribosomal protein S6 kinases (RSK), serum- and glucocorticoid-induced protein kinase (SGK) isoforms, and several protein kinase C (PKC) isoforms, by phosphorylating at the serine/threonine residues in their T-loop. [1]
The autophosphorylation of PDK1 at Ser241 in the T-loop or activation loop contributes to the catalytic activity of PDK1 upon substrate binding, and several other phosphorylation sites have also been proposed to contribute to the regulation of PDK1 activity. Upon growth factor stimulation and PtdIns(3,4,5)P3 production, both PKB/Akt and PDK1 translocate to the plasma membrane via the specific interaction of their PH domains with newly generated PtdIns(3,4,5)P3, where PDK1 could then readily phosphorylate and activate PKB/Akt, allowing PKB/Akt to phosphorylate downstream targets such as Foxo1. The mTORC1 or mTORC2 mediated phosphorylation of the hydrophobic motif of AGC kinases is essential for PDK1-mediated activation. This is achieved by creating a docking site for the binding of PDK1 to facilitate the phosphorylation of the kinases at its T-loop by PDK1. Moreover, the interaction of the hydrophobic motif binding pocket (PIF pocket) of PDK1 with the phosphorylated hydrophobic motif in the substrate induces the allosteric activation of PDK1. [1]
Therefore, through targeting a particular set of these AGC kinases, PDK1 play a critical role in regulating a wide variety of physiological processes including cell growth, proliferation, survival, development, and metabolism. PDK1 knockout mice die during early embryonic development, and the PDK1 dominant negative mutants knock-in mice are also shown to be embryonically lethal. A series of tissue-specific conditional knockout mice lacking PDK1 also confirm both the implication of PDK1 in mediating metabolic responses to insulin as well as in promoting cell viability, which proposes that selective activation of PDK1 might be a good strategy for the treatment of diabetes. However, PDK1 overexpression and amplification of the PDK1 gene are common occurrences in breast cancer and acute myeloid leukaemia. PDK1 also displays an epistatic relationship with the lipid phosphatase PTEN in the migration and malignant transformation of lymphocytes and in regulating nervous system development. Moreover, PTEN heterozygous mice expressing reduced levels of PDK1 are markedly protected from developing a wide range of tumors, indicating that PDK1 is a key effector in mediating tumorigenesis resulting from loss of PTEN and further validating PDK1 as a valuable anticancer target for the development of specific inhibitors. [1]
