DNA-dependent protein kinase (DNA-PK) is a nuclear protein Serine (Ser)/Threonine (Thr) kinase that acts as both a molecular sensor and transmitter of DNA damage, and plays important roles in the DNA repair of double stranded breaks (DSBs), mediating immunoglobulin V(D)J gene recombination events, as well as telomere stabilization. [show the full text]
DNA-PK Inhibitoren (DNA-PK Inhibitors)
| Kat.-Nr. | Produktname | Informationen | Publikationen | Validierung |
|---|---|---|---|---|
| S8586 | Nedisertib (M3814) | Nedisertib (M3814, Peposertib, MSC2490484A) ist ein oral bioverfügbarer, hochwirksamer und selektiver Inhibitor der DNA activated protein kinase (DNA-PK) mit einer IC50 von < 3 nM. |
|
|
| S8843 | AZD7648 | AZD7648 ist ein potenter Inhibitor von DNA-PK mit einer IC50 von 0,6 nM im biochemischen Assay und mehr als 100-fach selektiver gegenüber 396 anderen Kinasen. |
|
|
| S2638 | NU7441 (KU-57788) | NU7441 (KU-57788) ist ein hochwirksamer und selektiver DNA-PK-Inhibitor mit einer IC50 von 14 nM. Er hemmt auch mTOR und PI3K mit einer IC50 von 1,7 μM bzw. 5 μM in zellfreien Assays und reduziert die Häufigkeit von NHEJ, während die Rate der HDR nach Cas9-vermittelter DNA-Spaltung erhöht wird. |
|

|
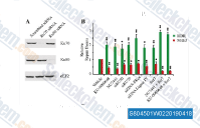
| S8045 | KU-0060648 | KU-0060648 ist ein dualer Inhibitor von DNA-PK und PI3Kα, PI3Kβ, PI3Kδ mit einer IC50 von 8,6 nM bzw. 4 nM, 0,5 nM, 0,1 nM, einer geringeren Hemmung von PI3Kγ mit einer IC50 von 0,59 μM. |
|
|
| S8593 | VX-984 | VX-984 (M9831) ist ein oral aktiver, potenter, selektiver und ATP-kompetitiver Inhibitor der DNA-PK. Diese Verbindung unterdrückt effektiv das nicht-homologe Endjoining (NHEJ) und erhöht DNA-Doppelstrangbrüche (DSBs). Es verstärkt in vitro die zytotoxischen Effekte ionisierender Strahlung (IR) in verschiedenen Krebszelllinien, einschließlich nicht-kleinzelliger Lungenkrebszelllinien (NSCLC). Zusätzlich reduziert dieser Inhibitor die DNA-PKcs-Autophosphorylierung. | ||
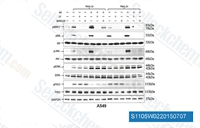
| S1105 | LY294002 | LY294002 (SF 1101, NSC 697286) ist das erste synthetische Molekül, das PI3Kα/δ/β mit einer IC50 von 0,5 μM/0,57 μM/0,97 μM hemmt; es ist in Lösung stabiler als Wortmannin und blockiert auch die Autophagosomenbildung. Es bindet nicht nur an Klasse-I-PI3Ks und andere PI3K-verwandte Kinasen, sondern auch an neuartige Ziele, die scheinbar nicht zur PI3K-Familie gehören. Diese Verbindung hemmt auch CK2 mit einer IC50 von 98 nM. Es ist ein nicht-spezifischer DNA-PKcs-Inhibitor und aktiviert Autophagy und Apoptosis. |
|
|
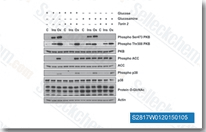
| S2817 | Torin 2 | Torin 2 ist ein potenter und selektiver mTOR-Inhibitor mit einer IC50 von 0,25 nM in p53 / - MEF-Zelllinien; 800-fach höhere Selektivität für mTOR als PI3K und verbesserte pharmakokinetische Eigenschaften. Diese Verbindung hemmt ATM/ATR/DNA-PK mit einer EC50 von 28 nM/35 nM/118 nM,in PC3-Zelllinien. Es verringert die Zellviabilität und induziert Autophagy und Apoptosis. |
|
|
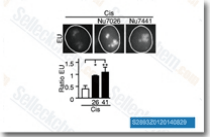
| S2893 | NU7026 | NU7026 (LY293646) ist ein potenter DNA-PK-Inhibitor mit einer IC50 von 0,23 μM in zellfreien Assays, 60-fach selektiver für DNA-PK als PI3K und inaktiv gegen sowohl ATM als auch ATR. Diese Verbindung verstärkt den G2/M-Zellzyklusarrest und die Apoptose. |
|
|
| S7891 | CC-115 | CC-115 ist ein dualer Inhibitor der DNA-abhängigen Proteinkinase (DNA-PK) und des Säugetier-Target of Rapamycin (mTOR) mit IC50-Werten von 0,013 μM bzw. 0,021 μM. Es hat eine potenzielle antineoplastische Aktivität. |
|
|
| S8379 | YU238259 | YU238259 ist ein neuartiger Inhibitor der Homologie-abhängigen DNA-Reparatur (HDR), der jedoch die nicht-homologe Endverknüpfung (NHEJ) in zellbasierten GFP-Reporter-Assays nicht hemmt. |
|
|
DNA-dependent protein kinase (DNA-PK) is composed of three key components including two DNA-binding subunits Ku70 and Ku80 (Ku86), as well as one DNA-dependent protein kinase catalytic subunit (DNA-PKcs). Based on protein sequence similarity, DNA-PK belongs to the phosphatidylinositol-3-kinase (PI3K) family, whereas, DNA-PK is not known to phosphorylate lipids and is therefore called PI3K-like kinase (PI3KK). The carboxyl-terminal region of Ku70 contains a SAP domain that is believed to be involved in chromosomal organization. The carboxyl-terminal region of Ku80 is required for the Ku70 and Ku80 heterodimer interaction with DNA-PKcs. The Ku heterodimer can bind to a variety of double-stranded end structures, including blunt ends, overhangs are the 3' or 5' end, and covalently closed hairpin ends. Like ATM and ATR, DNA-PKcs is structurally similar as it contains carboxyl-terminal domains, a large amino-terminal domain in addition to FAT and FATC domains flanking the kinase domain. The DNA-PKcs structure contains a channel large enough to accommodate double-stranded DNA, while the structure of Ku heterodimer is an asymmetric open ring, allowing the DNA to pass through the center. DNA-PKcs is one of the largest kinases identified to date, and it is the only kinase that is absolutely dependent on DNA binding for activity. DNA-PK has a strong preference for phosphorylating Serine (Ser) and Threonine (Thr) residues that are followed by glutamine or, less commonly, a hydrophobic residue. [1][2]
DNA-PK is involved in the ligation step of the non-homologous end joining (NHEJ) pathway required for DNA double-stranded break (DSB) repair, V(D)J recombination and telomere stabilization. A heterodimer of Ku70 and Ku80 initially binds to the double-stranded DNA broken ends and translocates inwards in an ATP-independent manner and recruits DNA-PKcs. This results in the stabilization of the protein/DNA binding and enabling NHEJ to proceed. Moreover, DNA-PKcs acts as a scaffold protein by joining two broken DNA ends together in a complex containing two DNA-PKcs molecules that contributes to the synapsis of the broken DNA ends and the localization of DNA repair proteins such as DNA ligase IV/XRCC4 complex to the site of damage. DNA-PK is activated in cis by the DNA to which it is bound, and stimulated by Ku heterodimer as well as the interaction of two molecules of DNA-PKcs, while end-bridging through synapsis is required for full kinase activation. DNA-PKcs autophosphorylation at multiple sites, including Thr2609 and Ser2056, results in an inactivation of DNA-PK kinase activity and NHEJ ability. To ensure NHEJ can proceed efficiently, DNA-PK phosphorylates and activates the Werner syndrome protein (WRN) to remove 3' phosphate or 3' phosphoglycolate groups generated following IR, and the nuclease Artemis to remove 5' overhangs and shorten 3' overhangs. In addition, DNA-PK promotes processing of hairpin DNA structures in V(D)J recombination by activation of Artemis. Cells that lack DNA-PKcs are acutely radiosensitive and have defective DSB repair, while mice lacking DNA-PKcs remain viable but are immunodeficient (due to the absence of immune development) as a result of accumulated processed DNA intermediates. Additionally, DNA-PK has been strongly implicated in telomere maintenance. DNA-PKcs-/- mice display significant telomeric fusion events consistent with the role of DNA-PKcs in telomere maintenance. Furthermore, DNA-PK is involved in the modulation of transcription by phosphorylation of RNA polymerases including pol I and pol II through its kinase activity, thereby regulating the function of these enzymes. By direct p53 phosphorylation, the modification of Ku70 releasing Bax, or suppressing the expression of p21, DNA-PK plays a significant role in mediating a p53-dependent apoptotic response under a range of cellular conditions including exposure to ionizing radiation (IR), environmental carcinogens and chemotherapeutic agents or in cells that have critically shortened telomeres. [1][2]
Specific inhibitors of DNA-PK used to selectively reduce NHEJ activity have been shown to be effective as single-agent therapies in homologous recombination (HR) -defective tumors. Treatment with a flavone-based DNA-PK inhibitor IC87361 leads to tumor regression. The inhibitors of DNA-PK such as NU7441 enhance the cytotoxicity of physical and chemical agents, leading to reduced clonogenic survival and cellular proliferation, as well as increased apoptosis, regardless of p53 status. Moreover, DNA-PK inhibitors combined with other DNA-damage response (DDR) inhibitors enhance the therapeutic potential of anticancer agents. [3][4]
