nur für Forschungszwecke
Daclatasvir (BMS-790052) HCV Protease Inhibitor
Kat.-Nr.S1482
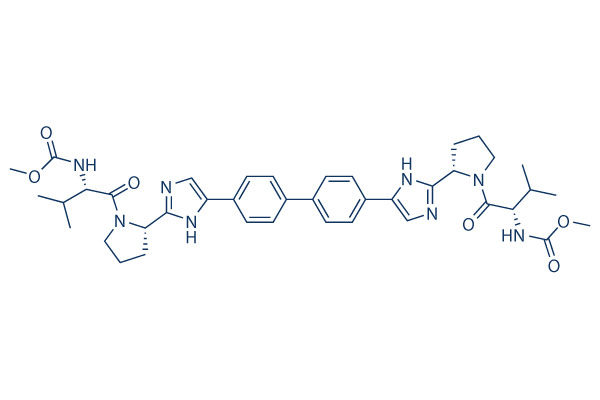
Chemische Struktur
Molekulargewicht: 738.88
Qualitätskontrolle
| Verwandte Ziele | HDAC Caspase Proteasome Secretase MMP Cysteine Protease DPP Tyrosinase HIV Protease Serine Protease |
|---|---|
| Weitere HCV Protease Inhibitoren | Danoprevir Lomibuvir (VX-222) Asunaprevir Tizoxanide PSI-6206 (GS-331007) Mecarbinate Tegobuvir Herba taxilli Extract 2'-C-Methylcytidine |
Zellkultur, Behandlung & Arbeitskonzentration
| Zelllinien | Assay-Typ | Konzentration | Inkubationszeit | Formulierung | Aktivitätsbeschreibung | PMID |
|---|---|---|---|---|---|---|
| human CEM cells | Cytotoxicity assay | 3 days | Cytotoxicity against human CEM cells after 3 days, CC50=9.6 μM | 25154714 | ||
| Huh7 | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1b infected in human Huh7 cells after 3 days by cell-based replicon assay, EC50=0.000003μM | 25148100 | ||
| Huh-luc/neo-ET replicon | Antiviral assay | 48 hrs | Antiviral activity against Hepatitis C virus genotype 1b harboring NS5A L28V mutant gene in Huh-luc/neo-ET replicon cells after 48 hrs by transient replicon mutant-based luciferase assay, EC50=0.000004μM | 24568313 | ||
| HuH7 | Antiviral assay | Antiviral activity against HCV genotype 1b infected in human HuH7 cells assessed as reduction in viral RNA replication, EC50=0.000004μM | 26099532 | |||
| HuH7 | Antiviral assay | Antiviral activity against HCV1b infected in human HuH7 cells assessed as inhibition of viral replication by RT-PCR analysis, EC50=0.0000066μM | 22507961 | |||
| HuH-Lcu-Neo | Function assay | 48 hrs | Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase reporter gene assay, EC50=0.0000081μM | 32202782 | ||
| Huh-5.2 | Antiviral assay | 4 days | Antiviral activity against Hepatitis C virus genotype 1b infected in human Huh-5.2 cells assessed as decrease in HCV replicon RNA replication after 4 days by luciferase assay, EC50=0.000009μM | 24900811 | ||
| HuH7 | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1b Con1 infected in human HuH7 cells assessed as inhibition of viral RNA replication after 3 days by renilla luciferase reporter gene assay, EC50=0.000009μM | 24320933 | ||
| HuH7 | Antiviral assay | 72 hrs | Antiviral activity against HCV1b infected in human HuH7 cells assessed as inhibition of viral replication after 72 hrs by FRET assay, EC50=0.000009μM | 24521299 | ||
| HuH7 | Antiviral assay | Antiviral activity against HCV genotype 1b Con1 infected in human HuH7 cells assessed as inhibition of viral replication, EC50=0.000009μM | 26077493 | |||
| Huh7.5/J6/JFH1/EMCVIRES/hRlucNeo | Function assay | 72 hrs | Inhibition of NS5A in HCV genotype 2a infected in human Huh7.5/J6/JFH1/EMCVIRES/hRlucNeo cells assessed as inhibition of replicon levels incubated for 72 hrs by luciferase reporter gene assay, IC50=0.000009μM | 31710479 | ||
| HuH7 replicon | Function assay | 2 days | Inhibition of NS5A in HCV genotype 1b infected in human HuH7 replicon cells assessed as reduction in subgenomic viral RNA replication treated for 2 days followed by compound washout and subsequent compound dosing measured after 1 day by SEAP reporter gene, EC50=0.000009μM | 30772607 | ||
| HuH7 | Antiviral assay | Antiviral activity against HCV 1b infected in human HuH7 cells by in vitro replicon assay, EC50=0.00001μM | 23466233 | |||
| Huh-luc/neo-ET replicon | Antiviral assay | 48 hrs | Antiviral activity against Hepatitis C virus genotype 1b in Huh-luc/neo-ET replicon cells after 48 hrs by replicon-based luciferase assay, EC50=0.00001μM | 24568313 | ||
| HuH7 | Function assay | Inhibition of NS5A in HCV genotype-1b infected in human HuH7 cells by luciferase reporter gene assay, EC50=0.000023μM | 25453810 | |||
| HuH7.5/Con1/SG-Neo(I)-hRluc2aUb | Function assay | 72 hrs | Inhibition of NS5A in HCV genotype 1b infected in human HuH7.5/Con1/SG-Neo(I)-hRluc2aUb cells assessed as inhibition of replicon levels incubated for 72 hrs by luciferase reporter gene assay, IC50=0.000023μM | 31710479 | ||
| HuH7 | Antiviral assay | Antiviral activity against HCV genotype 1b JFH-1 infected in human HuH7 cells assessed as inhibition of viral replication, EC50=0.000028μM | 26077493 | |||
| HuH7 | Antiviral assay | Antiviral activity against HCV 1b infected in human HuH7 cells by in vitro replicon assay, EC90=0.00003μM | 23466233 | |||
| HuH-Lcu-Neo | Function assay | 48 hrs | Inhibition of NS5A in HCV genotype 1a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase reporter gene assay, EC50=0.0000324μM | 32202782 | ||
| HuH7 | Antiviral assay | Antiviral activity against HCV genotype 1b expressing NS5A L31V mutant infected in human HuH7 cells assessed as reduction in viral RNA replication, EC50=0.000035μM | 26099532 | |||
| Huh-luc/neo-ET replicon | Antiviral assay | 48 hrs | Antiviral activity against Hepatitis C virus genotype 1a in Huh-luc/neo-ET replicon cells after 48 hrs by replicon-based luciferase assay, EC50=0.0000398μM | 24568313 | ||
| HuH7 | Antiviral assay | Antiviral activity against wild type HCV genotype 1b infected in human HuH7 cells assessed as reduction in viral RNA replication, EC50=0.000048μM | 26099532 | |||
| W11.8 | Antiviral assay | 4 days | Antiviral activity against Hepatitis C virus genotype 1a infected in W11.8 cells assessed as decrease in NS5A expression in replicon cell after 4 days by luminescence based ELISA, EC50=0.00005μM | 24900811 | ||
| HuH7 | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1a H77 infected in human HuH7 cells assessed as inhibition of viral RNA replication after 3 days by renilla luciferase reporter gene assay, EC50=0.00005μM | 24320933 | ||
| HuH7 | Antiviral assay | 72 hrs | Antiviral activity against HCV1a infected in human HuH7 cells assessed as inhibition of viral replication after 72 hrs by FRET assay, EC50=0.00005μM | 24521299 | ||
| HuH7 | Antiviral assay | Antiviral activity against HCV genotype 5a infected in human HuH7 cells by luciferase reporter gene assay, EC50=0.000051μM | 25453811 | |||
| HuH7 | Antiviral assay | Antiviral activity against HCV genotype 1b infected in human HuH7 cells by luciferase reporter gene assay, EC50=0.000073μM | 25453811 | |||
| HuH-Lcu-Neo | Function assay | 48 hrs | Inhibition of NS5A in patient-derived HCV genotype 3a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase reporter gene assay, EC50=0.0001145μM | 32202782 | ||
| Huh-luc/neo-ET replicon | Antiviral assay | 48 hrs | Antiviral activity against Hepatitis C virus genotype 1b harboring NS5A L31V mutant gene in Huh-luc/neo-ET replicon cells after 48 hrs by transient replicon mutant-based luciferase assay, EC50=0.0001259μM | 24568313 | ||
| HuH7 | Function assay | Inhibition of NS5A in HCV genotype-1a infected in human HuH7 cells by luciferase reporter gene assay, EC50=0.00014μM | 25453810 | |||
| HuH7 | Antiviral assay | Antiviral activity against HCV genotype 1a infected in human HuH7 cells by luciferase reporter gene assay, EC50=0.00014μM | 25453811 | |||
| HuH7 | Antiviral assay | Antiviral activity against HCV genotype 1b expressing NS5A Y93H mutant infected in human HuH7 cells assessed as reduction in viral RNA replication, EC50=0.00018μM | 26099532 | |||
| Huh-luc/neo-ET replicon | Antiviral assay | 48 hrs | Antiviral activity against Hepatitis C virus genotype 1b harboring NS5A Y93H mutant gene in Huh-luc/neo-ET replicon cells after 48 hrs by transient replicon mutant-based luciferase assay, EC50=0.0003162μM | 24568313 | ||
| HuH7 | Antiviral assay | 72 hrs | Antiviral activity against HCV1a infected in human HuH7 cells assessed as inhibition of viral replication after 72 hrs by FRET assay, EC90=0.00038μM | 24521299 | ||
| HuH7 | Antiviral assay | Antiviral activity against HCV genotype 4a infected in human HuH7 cells by luciferase reporter gene assay, EC50=0.00041μM | 25453811 | |||
| HuH-Lcu-Neo | Function assay | 48 hrs | Inhibition of NS5A in HCV genotype 3a con infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase reporter gene assay, EC50=0.0004115μM | 32202782 | ||
| HuH7 | Antiviral assay | Antiviral activity against HCV genotype 6a infected in human HuH7 cells by luciferase reporter gene assay, EC50=0.00045μM | 25453811 | |||
| HuH7 | Antiviral assay | Antiviral activity against HCV genotype 3a infected in human HuH7 cells by luciferase reporter gene assay, EC50=0.00067μM | 25453811 | |||
| HuH7 | Antiviral assay | Antiviral activity against HCV genotype 2a infected in human HuH7 cells by luciferase reporter gene assay, EC50=0.00067μM | 25453811 | |||
| HuH-Lcu-Neo | Function assay | 48 hrs | Inhibition of NS5A in patient-derived HCV genotype 2a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase reporter gene assay, EC50=0.0494μM | 32202782 | ||
| HuH-Lcu-Neo | Function assay | 48 hrs | Inhibition of NS5A in HCV genotype 2a con infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase reporter gene assay, EC50=0.05285μM | 32202782 | ||
| CEM | Cytotoxicity assay | 3 days | Cytotoxicity against human CEM cells after 3 days, CC50=9.6μM | 22507961 | ||
| CEM | Cytotoxicity assay | Cytotoxicity against human CEM cells, CC50=9.6μM | 22704887 | |||
| Vero | Cytotoxicity assay | Cytotoxicity against african green monkey Vero cells, CC50=9.6μM | 23466233 | |||
| CEM | Cytotoxicity assay | Cytotoxicity against human CEM cells, CC50=10μM | 26099532 | |||
| PBMC | Cytotoxicity assay | Cytotoxicity against human PBMC cells, CC50=19μM | 22704887 | |||
| Vero | Cytotoxicity assay | 3 days | Cytotoxicity against african green monkey Vero cells after 3 days, CC50=21μM | 22507961 | ||
| Vero | Cytotoxicity assay | Cytotoxicity against african green monkey Vero cells, CC50=21μM | 22704887 | |||
| CEM | Cytotoxicity assay | Cytotoxicity against human CEM cells, CC50=21μM | 23466233 | |||
| Vero | Cytotoxicity assay | Cytotoxicity against african green monkey Vero cells, CC50=21μM | 26099532 | |||
| Huh7.5.1 | Antiviral assay | 100 pM to 1 uM | Antiviral activity against HCV genotype 1b NK/R2AN infected in human Huh7.5.1 cells expressing NS5A L31V mutant assessed as reduction in virus replication at 100 pM to 1 uM by luciferase reporter gene assay | 26134551 | ||
| Huh7.5.1 | Antiviral assay | 100 pM to 1 uM | Antiviral activity against HCV genotype 1b NK/R2AN infected in human Huh7.5.1 cells expressing NS5A Y93H mutant assessed as reduction in virus replication at 100 pM to 1 uM by luciferase reporter gene assay | 26134551 | ||
| GS4.3 | Antiviral assay | 0.15 uM | 6 days | Antiviral activity against HCV infected in human GS4.3 cells assessed as inhibition of NS3/4A levels at 0.15 uM treated with fresh media containing compound every 2 days measured after 6 days by Western blot method | 29232582 | |
| GS4.3 | Antiviral assay | 0.15 uM | 6 days | Antiviral activity against HCV infected in human GS4.3 cells assessed as inhibition of viral core protein levels at 0.15 uM treated with fresh media containing compound every 2 days measured after 6 days by Western blot method | 29232582 | |
| HuH7 | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1b infected in human HuH7 cells at >= 10 times antiviral EC50 after 3 days by luciferase reporter assay | 28430437 | ||
| HuH7 | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1b infected in human HuH7 cells co-treated with asunaprevir after 3 days by luciferase reporter assay | 28430437 | ||
| Klicken Sie hier, um weitere experimentelle Daten zu Zelllinien anzuzeigen | ||||||
Chemische Informationen, Lagerung & Stabilität
| Molekulargewicht | 738.88 | Formel | C40H50N8O6 |
Lagerung (Ab dem Eingangsdatum) | |
|---|---|---|---|---|---|
| CAS-Nr. | 1009119-64-5 | SDF herunterladen | Lagerung von Stammlösungen |
|
|
| Synonyme | EBP883 | Smiles | CC(C)C(C(=O)N1CCCC1C2=NC=C(N2)C3=CC=C(C=C3)C4=CC=C(C=C4)C5=CN=C(N5)C6CCCN6C(=O)C(C(C)C)NC(=O)OC)NC(=O)OC | ||
Löslichkeit
|
In vitro |
DMSO
: 148 mg/mL
(200.3 mM)
Ethanol : 148 mg/mL Water : Insoluble |
Molaritätsrechner
|
In vivo |
|||||
In-vivo-Formulierungsrechner (Klare Lösung)
Schritt 1: Geben Sie die untenstehenden Informationen ein (Empfohlen: Ein zusätzliches Tier zur Berücksichtigung von Verlusten während des Experiments)
Schritt 2: Geben Sie die In-vivo-Formulierung ein (Dies ist nur der Rechner, keine Formulierung. Bitte kontaktieren Sie uns zuerst, wenn es im Abschnitt "Löslichkeit" keine In-vivo-Formulierung gibt.)
Berechnungsergebnisse:
Arbeitskonzentration: mg/ml;
Methode zur Herstellung der DMSO-Stammlösung: mg Wirkstoff vorgelöst in μL DMSO ( Konzentration der Stammlösung mg/mL, Bitte kontaktieren Sie uns zuerst, wenn die Konzentration die DMSO-Löslichkeit der Wirkstoffcharge überschreitet. )
Methode zur Herstellung der In-vivo-Formulierung: Nehmen Sie μL DMSO Stammlösung, dann hinzufügenμL PEG300, mischen und klären, dann hinzufügenμL Tween 80, mischen und klären, dann hinzufügen μL ddH2O, mischen und klären.
Methode zur Herstellung der In-vivo-Formulierung: Nehmen Sie μL DMSO Stammlösung, dann hinzufügen μL Maisöl, mischen und klären.
Hinweis: 1. Bitte stellen Sie sicher, dass die Flüssigkeit klar ist, bevor Sie das nächste Lösungsmittel hinzufügen.
2. Achten Sie darauf, das/die Lösungsmittel der Reihe nach hinzuzufügen. Sie müssen sicherstellen, dass die bei der vorherigen Zugabe erhaltene Lösung eine klare Lösung ist, bevor Sie mit der Zugabe des nächsten Lösungsmittels fortfahren. Physikalische Methoden wie Vortex, Ultraschall oder ein heißes Wasserbad können zur Unterstützung des Lösens verwendet werden.
Wirkmechanismus
| Merkmale |
First-in-class, highly selective inhibitor of hepatitis C virus (HCV) NS5A with picomolar EC50 values.
|
|---|---|
| Targets/IC50/Ki |
HCV NS5A
9 pM-50 pM(EC50)
|
| In vitro |
Daclatasvir (BMS-790052) ist einer der bisher potentesten Inhibitoren der HCV-Replikation. Die mittleren EC50-Werte dieser Verbindung betragen 50 und 9 pM für HCV-Genotyp 1a bzw. 1b Replikone. Es zeigt einen therapeutischen Index (CC50/EC50) von mindestens 105 und ist inaktiv gegenüber einer Reihe von 10 RNA- und DNA-Viren, mit EC50 höher als 10 μM. Dies bestätigt seine Spezifität für HCV.
In Huh7-Zellen, die die HCV-Genotyp-1b-Replikone beherbergen, blockiert es sowohl die transiente als auch die stabile HCV-Genomreplikation mit EC50-Werten im Bereich von 1-15 pM. Bei Konzentrationen von 100 pM oder 1 nM wurde gezeigt, dass es die subzelluläre Lokalisation und biochemische Fraktionierung von NS5A verändert.
Diese Verbindung hemmt Hybridreplikone, die HCV-Genotyp-4 NS5A-Gene enthalten, mit einer EC50 von 7-13 pM. Residue 30 von NS5A ist eine wichtige Stelle für die BMS-790052-vermittelte Resistenz in den Hybridreplikonen.
|
| Kinase-Assay |
FRET-Assay für HCV NS5A-Inhibitoren
|
|
Das Peptid (Ac-Asp-Glu-Asp [EDANS]-Glu-Glu-Abu-[COO] Ala-Ser-Lys [DABCYL]-NH2) enthält einen Fluoreszenzdonor {EDANS, 5-[(2-Aminoethyl)amino]naphthalin-1-sulfonsäure} in der Nähe eines Endes und einen Akzeptor {DABCYL, 4-[(4-Dimethylamino)phenyl]azo}benzoesäure} in der Nähe des anderen Endes. Der intermolekulare Resonanzenergietransfer zwischen Donor und Akzeptor löscht die Fluoreszenz des Peptids, aber wenn die NS3-Protease das Peptid spaltet, werden die Produkte aus der Resonanzenergietransferlöschung freigesetzt. Wenn mehr Substrat durch die NS3-Protease gespalten wird, nimmt die Fluoreszenz des Donors mit der Zeit zu. Die Nachweisreagenzien sind: 5× Luciferase-Zellkultur-Lysereagenz, mit dH2O auf 1× verdünnt, Zugabe von NaCl (150 mM) und FRET-Peptid (20 μM). HCV-Huh-7-Zellen wurden in 96-Well-Platten gegeben und über Nacht haften gelassen (1×104 Zellen pro Well). Am nächsten Tag wurde Daclatasvir (BMS-790052) in die Wells gegeben und die Platte 72 Stunden inkubiert. Anschließend wurden die Platten mit PBS gewaschen und ein FRET-Assay durch Zugabe von 30 μL der oben genannten FRET-Peptid-Nachweisreagenzien zu jedem Well durchgeführt. Die Signale wurden mit einem Cytofluor 4000-Gerät erfasst, das auf 340 nm (Anregung)/490 nm (Emission) im Automatikmodus eingestellt war, 20 Zyklen oder weniger lief und die Platte im kinetischen Modus ablas. Nach dem FRET-Assay wurden 40 μL Luciferase-Substrat zu jedem Well gegeben und die Luciferase-Aktivität gemessen.
|
Literatur |
|
Klinische Studieninformationen
(Daten von https://clinicaltrials.gov, aktualisiert am 2024-05-22)
| NCT-Nummer | Rekrutierung | Erkrankungen | Sponsor/Kooperationspartner | Startdatum | Phasen |
|---|---|---|---|---|---|
| NCT05992077 | Recruiting | HCV Infection |
ANRS Emerging Infectious Diseases |
August 7 2023 | Not Applicable |
| NCT04852614 | Recruiting | Hepatitis C Virus Infection |
Ain Shams University |
December 1 2020 | -- |
| NCT04773756 | Completed | Covid19 |
Alexandria University |
November 1 2020 | Phase 4 |
| NCT03208322 | Withdrawn | Hepatitis C |
Bristol-Myers Squibb |
November 30 2018 | -- |
Technischer Support
Tel: +1-832-582-8158 Ext:3
Wenn Sie weitere Fragen haben, hinterlassen Sie bitte eine Nachricht.
