nur für Forschungszwecke
Brefeldin A (BFA chemical) Proteintransport-Inhibitor
Kat.-Nr.S7046
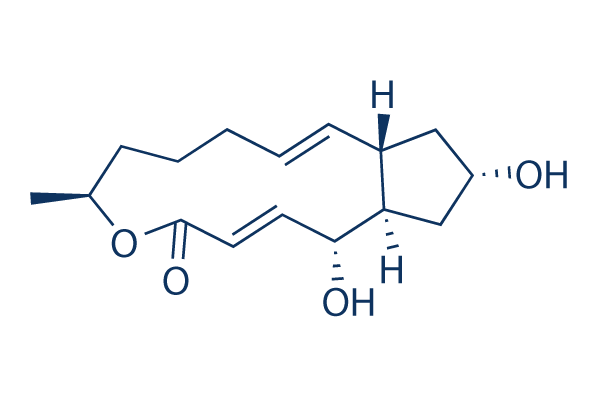
Chemische Struktur
Molekulargewicht: 280.36
Qualitätskontrolle
| Verwandte Ziele | CFTR CRM1 CD markers AChR Calcium Channel Sodium Channel Potassium Channel GABA Receptor TRP Channel GluR |
|---|---|
| Weitere ATPase Inhibitoren | (-)-Blebbistatin Thapsigargin CB-5083 Golgicide A Sodium orthovanadate Oleic Acid Bufalin Ginsenoside Rb1 CDN1163 BTB06584 |
Zellkultur, Behandlung & Arbeitskonzentration
| Zelllinien | Assay-Typ | Konzentration | Inkubationszeit | Formulierung | Aktivitätsbeschreibung | PMID |
|---|---|---|---|---|---|---|
| PC12 | Function Assay | 2 μM | 1 h | inhibits the L-DOPA (20 μM)-induced transient ERK1/2 phosphorylation | 26363191 | |
| C2C12 | Function Assay | 1 μg/ml | 1 h | abolishes cytokine release from C2C12 myotubes | 26291279 | |
| MEFs WT | Function Assay | 5 μM | 20 min | causes resident enzymes such as NAGT-GFP, to diffuse back to the ER | 26196023 | |
| MEFs VAMP7 KO | Function Assay | 5 μM | 20 min | causes resident enzymes such as NAGT-GFP, to diffuse back to the ER | 26196023 | |
| SMCs | Function Assay | 10 µg/ml | 0-12 h | DMSO | shows a trend towards a higher concentration of the ER/SR network in the perinuclear area | 26172080 |
| SMCs | Function Assay | 10 µg/ml | 0-12 h | DMSO | causes a transient Ca2+ release from the ER/SR | 26172080 |
| HEMC-1 | Function Assay | 0.1 µg/ml | 24 h | causes a higher inhibitory effect on exocytosis than nocodazole | 25972759 | |
| HUVEC | Function Assay | 10 μM | 1 h | DMSO | abolishes hypoxia-induced release of ATP from apical and basolateral surfaces | 25956988 |
| HUVEC | Function Assay | 10 μM | 1 h | DMSO | increases the number and intensity of fluorescent areas especially in perinuclear space | 25956988 |
| Caco-2 | Function Assay | 2.5 μM | 30 min | attenuates the TGF-β1-mediated increase in SERT function | 25954931 | |
| NRK | Function Assay | 200 ng/ml | 4 h | DMSO | rescues mitotic progression | 25948586 |
| HeLa | Function Assay | 200 ng/ml | 3 h | DMSO | induces the artificial break-up of the Golgi complex | 25948586 |
| COS | Function Assay | 1 μg/ml | 3 h | completely disperses the AP-1 signal | 25915900 | |
| DF1 | Function Assay | 1 μM | 48 h | DMSO | disperses the exogenous CSGalNAcT2 protein | 25807054 |
| nHDFs | Function Assay | 1 μM | 2 h | prevents the assembly of cytosolic coat proteins onto Golgi membranes | 25772616 | |
| FRT | Function Assay | 5 μg/ml | 2 h | blocks trafficking through the Golgi complex by inhibiting ER-to-Golgi transport | 25767115 | |
| FRT | Function Assay | 5 μg/ml | 2 h | prevents the increase in cleaved α subunits when [Na+]i was reduced | 25767115 | |
| HepG2 | Function Assay | 1 µM | 24 h | DMSO | decreases the level of PXR mRNA | 25616597 |
| SMCs | Function Assay | 1μg/mL | 3 h | accumulates CNPY2 protein in the ER compartment and no longer co-localized with the Golgi marker | 25589425 | |
| OB-6 | Apoptosis Assay | 2.7 μM | 48 h | induces apoptosis | 25532480 | |
| iPSC-CMs | Function Assay | 500 ng/ml | 48 h | increases the intensity of the higher mobility LAMPs at the cost of the lower mobility species | 25488666 | |
| SP-Nluc | Function Assay | 5 mg/mL | 6 h | DMSO | causes an increase in reporter activity in the parasite | 25392998 |
| PEXEL-Nluc | Function Assay | 5 mg/mL | 6 h | DMSO | causes an increase in reporter activity in the parasite | 25392998 |
| H1299 | Function Assay | 10 μg/ml | 24 h | induces autophagy | 25388970 | |
| MDA-MB-231 | Cell Viability Assay | 0–50 μg/mL | 48 h | EC50 = 0.016 µg/mL | 25356567 | |
| MDA-MB-231 | Apoptosis Assay | 0.1 μg/mL | 4 h | induces apoptosis | 25356567 | |
| MDA-MB-231 | Growth Inhibition Assay | 0.01/0.05 μg/mL | 24 h | increases the fraction of sub-G1 cell debris | 25356567 | |
| MDA-MB-231 | Apoptosis Assay | 0.05–1 μg/mL | 24 h | induces PARP (poly ADP-ribose polymerase-1) cleavage | 25356567 | |
| MDA-MB-231 | Function Assay | 0–50 μg/mL | 24 h | inhibits the formation of 3D and 2D colonies | 25356567 | |
| A172 | Function Assay | 10 μg/ml | 4 h | DMSO | results in the retrograde transport of fluorescent granules | 25239507 |
| KMS-6 | Function Assay | 1 μM | 24 h | exhibits half the secretion of galanin-LI as did the control | 25229126 | |
| MEC | Function Assay | 1 μM | 1.5 h | causes a dramatic decrease in the surface VEGFR2 | 25228815 | |
| HEK293/hERG | Function Assay | 10 μM | 1 h | results in a time-dependent reduction mature hERG protein | 25218469 | |
| RBE4 | Apoptosis Assay | 2 μM | 3–24 h | induces apoptosis time dependently | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | increases the XBP1 protein levels after 3 and 6 h of treatment | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | increases active caspase-12 in a time-dependent manner | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | increases the levels of ROS time-dependently | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | induces a delayed depletion of the ER Ca2+ content at 6 h of incubation significantly | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | induces an overload of Ca2+ in the mitochondria in the first 6 h of incubation (p < 0.001) but Ca2+ levels in this organelle decreased after 12 h of incubation | 25128025 | |
| Huh-7 | Function Assay | 1μg/mL | 3–24 h | increases the level of APE1 in a time-dependent manner | 25026174 | |
| HepG2 | Function Assay | 1μg/mL | 3–24 h | increases the level of APE1 in a time-dependent manner | 25026174 | |
| H838-LKB1 | Function Assay | 30 ng/ml | 12/18 h | increases the protein levels of BiP | 25011082 | |
| H838-KDLKB1 | Function Assay | 30 ng/ml | 12/18 h | increases the protein levels of BiP | 25011082 | |
| H838-KDLKB1 | Function Assay | 30 ng/ml | 12/18 h | increases the levels of phosphorylated eIF2α (phospho-eIF2α) | 25011082 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 30 min | mimics the effects of insulin and causes robust phosphorylation of Akt (Ser 473) and phosphorylation of AS160 (Thr 642 and Ser 588) | 24843827 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 30 min | recapitulates insulin action with respect to regulating Akt activity and AS160 phosphorylation | 24843827 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 30 min | causes reversible redistribution of GLUT4 | 24843827 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 1 h | causes redistribution of GLUT4 but not increase in glucose uptake | 24843827 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 1 h | causes phosphorylation of the FoxO1 transcription factor | 24843827 | |
| HeLa | Function Assay | 5 μg/ml | 3 h | causes nuclear exclusion of the FoxO1 transcription factor and decreases transcription of FoxO1-regulated genes | 24843827 | |
| HEK293 | Function Assay | 5 μg/ml | 12 h | abolishes CMA-induced CRELD2 secretion | 24687431 | |
| COS-1 | Function Assay | 5 µg/ml | 24 h | restricts localization of NB in the perinuclear region | 24671751 | |
| PRP | Function Assay | 10 μM | abrogates SDF-1α-mediated CXCR7 externalization | 24668750 | ||
| RAW264.7 | Apoptosis Assay | 4 μM | 48 h | attenuates the inhibition of ox-LDL-induced apoptosis and the facilitation of cholesterol efflux by Ac-hE-18A-NH2 | 24639032 | |
| MDMs | Apoptosis Assay | 10 μg/ml | 12/15 h | induces apoptosis | 24556695 | |
| PMHs | Function Assay | 10–20 μg/ml | 24 h | DMSO | induced ER stress | 24407242 |
| PMHs | Apoptosis Assay | 10–20 μg/ml | 24 h | DMSO | increases cell death | 24407242 |
| HEK293/tau | Function Assay | 5 μM | 1/2/4 h | induces Golgi fragmentation | 24368089 | |
| HEK293/tau | Function Assay | 5 μM | 3 h | induces tau hyperphosphorylation | 24368089 | |
| ADF | Function Assay | 10 μM | 16 h | inhibits the ZnCl2-induced translocation of CRT | 24228232 | |
| U373 | Function Assay | 10 μM | 16 h | inhibits the ZnCl2-induced translocation of CRT | 24228232 | |
| RKO-HIPK2i | Function Assay | 10 μM | 16 h | inhibits the ZnCl2-induced translocation of CRT | 24228232 | |
| ADF | Function Assay | 10 μM | 6 h | impairs the DC activation | 24228232 | |
| Huh7 | Function Assay | 5 μg/ml | 4 h | abolishes the secretion of intracellular ApoB | 24100140 | |
| Huh7 | Function Assay | 5 μg/ml | 1 h | causes a significant increase in ApoB-crescents | 24100140 | |
| Huh7 | Function Assay | 5–10 ng/ml | 12 h | increases ApoB-crescents without inhibiting secretion | 24100140 | |
| BAECs | Function Assay | 5 μg/ml | 0-4 h | induces the rapid dephosphorylation of eNOS at Ser1179 | 24085225 | |
| Macrophages | Function Assay | 71 µM | 6 h | inhibits lunasin internalization | 24039740 | |
| Colo 205 | Growth Inhibition Assay | 0-5 μg/mL | 48 h | inhibits cell growth in suspension cultures with an estimated IC50 of ~15 ng/mL | 23973996 | |
| Colo 205 | Function Assay | 0.012-0.025 μg/mL | 14 d | reduces the clonogenicity of Colo 205 CSCs | 23973996 | |
| Colo 205 | Apoptosis Assay | 0.1 μg/mL | 0-24 h | induces apoptosis of Colo 205 cells in suspension cultures | 23973996 | |
| Colo 205 | Function Assay | 0.015 μg/mL | 24 h | induces the expression of ER stress-related genes | 23973996 | |
| Colo 205 | Function Assay | 0.015 μg/mL | 24 h | inhibits the activity of MMPs | 23973996 | |
| IBRS2 | Function Assay | 5 μg/ml | 0.5 h | DMSO | disrupts the ERGIC and Golgi | 23963534 |
| IBRS2 | Function Assay | 5 μg/ml | 0.5 h | DMSO | enhances FMDV infection | 23963534 |
| HeLa | Function Assay | 2 μM | 2 h | attenuates the TNF-induced secretion of IL-15 | 23950892 | |
| HFS | Function Assay | 0-1 μg/ml | 24 h | GLTP expression reaches a plateau at concentrations as low as 0.01 µg/ml | 23894633 | |
| HFS | Function Assay | 0.01 µg/ml | 24 h | increases the expression of glycosphingolipid synthase genes at 6 h | 23894633 | |
| OVCAR-3 | Growth Inhibition Assay | 1–15 μM | 24 h | induces a loss of cell viability dose dependently | 23826964 | |
| OVCAR-3 | Function Assay | 1–15 μM | 24 h | induces nuclear damage | 23826964 | |
| OVCAR-3 | Apoptosis Assay | 1-10 μM | 4 h | induces the activation of apoptosis-related proteins | 23826964 | |
| OVCAR-3 | Apoptosis Assay | 10 μM | 24 h | induces activation of caspases | 23826964 | |
| OVCAR-3 | Function Assay | 1–10 μM | 24 h | induces disruption of the mitochondrial transmembrane potential | 23826964 | |
| OVCAR-3 | Function Assay | 1–10 μM | 24 h | induces formation of reactive oxygen species | 23826964 | |
| OVCAR-3 | Function Assay | 1–10 μM | 24 h | inhibits cell adhesion and migration | 23826964 | |
| MKN45 | Growth Inhibition Assay | IC50<0.001 μg/ml | 23793342 | |||
| LOVO | Growth Inhibition Assay | IC50=0.12 μg/ml | 23793342 | |||
| A549 | Growth Inhibition Assay | IC50=0.04 μg/ml | 23793342 | |||
| MDA-MB-435 | Growth Inhibition Assay | IC50<0.001 μg/ml | 23793342 | |||
| HepG2 | Growth Inhibition Assay | IC50<0.001 μg/ml | 23793342 | |||
| HL-60 | Growth Inhibition Assay | IC50<0.001 μg/ml | 23793342 | |||
| neural precursor cells | Function assay | Inhibition of neurosphere proliferation of mouse neural precursor cells by MTT assay | 17417631 | |||
| HeLa | Function assay | 100 uM | 2 hrs | Dispersion of cis golgi marker betaCoP in human HeLa cells at 100 uM for 2 hrs | 17563369 | |
| HeLa | Function assay | 100 uM | 2 hrs | Dispersion of cis golgi marker KDEL in human HeLa cells at 100 uM for 2 hrs | 17563369 | |
| Vero | Function assay | 10 ug/ml | 5 mins | Inhibition of Arf1 in african green monkey Vero cells assessed as rapid AP-1 dispersal from golgi membranes at 10 ug/ml after 5 mins by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 5 mins | Inhibition of Arf1 in african green monkey Vero cells assessed as rapid GGA3 dispersal from trans golgi network at 10 ug/ml after 5 mins by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 uM | 1 hr | Inhibition of GBF1 QNV deleted mutant in african green monkey Vero cells assessed as effect on change in golgi morphology at 10 uM after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 uM | 1 hr | Inhibition of GBF1 QNV to AAA mutant in african green monkey Vero cells assessed as effect on change in golgi morphology at 10 uM after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as decrease in Arf1-GTP levels at 10 ug/ml after 1 hr | 19182783 | |
| Vero | Function assay | 10 uM | Inhibition of GBF1 in african green monkey Vero cells assessed as inhibition of StxB-SS retrogade transport from endosomes to TGN at 10 uM by immunofluorescence method | 19182783 | ||
| Vero | Function assay | 10 uM | 1 hr | Inhibition of GBF1 in african green monkey Vero cells assessed as punctate and diffuse distribution of medial-Golgi marker giantin from TGN at 10 uM after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as punctate and diffuse distribution of medial-Golgi marker giantin at 10 ug/ml after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 5 mins | Inhibition of Arf1 in african green monkey Vero cells assessed as rapid COPI redistribution from golgi at 10 ug/ml after 5 mins by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as tubule formation from trans golgi network and endosomes before its dispersal at 10 ug/ml after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as giantin positive punctate structures in contact with Sec31-positive ER exit site at 10 ug/ml after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | Inhibition of Arf1 in african green monkey Vero cells assessed as inhibition of StxB-SS retrogade transport from endosomes to TGN at 10 ug/ml by immunofluorescence method | 19182783 | ||
| Vero | Function assay | 10 uM | 1 hr | Induction of GBF1 in african green monkey Vero cells assessed as punctate and diffuse distribution of cis-Golgi marker GM130 from TGN at 10 uM after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as punctate and diffuse distribution of cis-Golgi marker GM130 at 10 ug/ml after 1 hr by immunofluorescence method | 19182783 | |
| NRK | Function assay | 7 uM | 60 mins | Golgi-disturbing activity in golgi apparatus of rat NRK cells assessed as fusion of golgi membrane fusion with endoplasmic reticulum at 7 uM after 60 mins by Hoechst 3342 staining-based immunofluorescence microscopy | 20189813 | |
| NCI60 | Cytostatic assay | Cytostatic activity against human NCI60 cells by SRB assay, GI50=0.0206μM. | 23805957 | |||
| NCI60 | Cytostatic assay | Cytostatic activity against human NCI60 cells by SRB assay, TGI=3.48μM. | 23805957 | |||
| HeLa R19 | Antiviral assay | 0.5 uM | 7 hrs | Antiviral activity against Coxsackievirus B3 infected in human HeLa R19 cells assessed as inhibition of viral replication at 0.5 uM after 7 hrs by luciferase reporter gene assay | 23805957 | |
| HeLa | Function assay | 5 uM | 30 to 60 mins | Induction of golgi apparatus disassembly in human HeLa cells at 5 uM after 30 to 60 mins by confocal microscopic analysis | 23805957 | |
| Arabidopsis thaliana root cells | Function assay | 90 uM | 30 mins | Induction of morphological changes of golgi apparatus in Arabidopsis thaliana root cells expressing ST-YFP/VHAa1-RFP at 90 uM after 30 mins by confocal laser scanning microscopic analysis | 23805957 | |
| HeLa R19 | Antiviral assay | 5 to 50 uM | 7 hrs | Antiviral activity against Coxsackievirus B3 infected in human HeLa R19 cells assessed as inhibition of viral replication at 5 to 50 uM after 7 hrs by luciferase reporter gene assay | 23805957 | |
| PC3 | Function assay | 50 nM | 72 hrs | Potentiation of 3 nM docetaxel-induced cytotoxicity against human PC3 cells assessed as decrease in cell viability at 50 nM after 72 hrs by trypan blue exclusion assay | 28462831 | |
| L02 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human L02 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50<0.0004μM. | 28494251 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50=0.068μM. | 28494251 | ||
| HT-29 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT-29 cells after 72 hrs by MTT assay, IC50=0.16μM. | 28494251 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by MTT assay, IC50=0.35μM. | 28494251 | ||
| LO2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human LO2 cells after 72 hrs by MTT assay, IC50<0.001μM. | 29524728 | ||
| Bel7402 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402 cells after 72 hrs by MTT assay, IC50=0.024μM. | 29524728 | ||
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells after 72 hrs by MTT assay, IC50=0.025μM. | 29524728 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50=0.068μM. | 29524728 | ||
| Bel7402/5-FU | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402/5-FU cells after 72 hrs by MTT assay, IC50=0.82μM. | 29524728 | ||
| HeLa | Function assay | 18 uM | 3 hrs | Inhibition of alkaline phosphatase secretion in human HeLa cells at 18 uM incubated for 3 hrs | 31421965 | |
| VERO-E6 | Function assay | 48 hrs | Determination of IC50 values for inhibition of SARS-CoV-2 induced cytotoxicity of VERO-E6 cells after 48 hours exposure to 0.01 MOI SARS CoV-2 virus by high content imaging, IC50=0.02μM. | ChEMBL | ||
| VERO-E6 | Function assay | 48 hrs | Toxicity CC50 against VERO-E6 cells determined at 48 hours by high content imaging (same conditions as 2_LEY without exposure to 0.01 MOI SARS CoV-2 virus), CC50=0.06μM. | ChEMBL | ||
| Klicken Sie hier, um weitere experimentelle Daten zu Zelllinien anzuzeigen | ||||||
Chemische Informationen, Lagerung & Stabilität
| Molekulargewicht | 280.36 | Formel | C16H24O4 |
Lagerung (Ab dem Eingangsdatum) | |
|---|---|---|---|---|---|
| CAS-Nr. | 20350-15-6 | SDF herunterladen | Lagerung von Stammlösungen |
|
|
| Synonyme | Cyanein, Decumbin | Smiles | CC1CCCC=CC2CC(CC2C(C=CC(=O)O1)O)O | ||
Löslichkeit
|
In vitro |
DMSO
: 56 mg/mL
(199.74 mM)
Water : Insoluble Ethanol : Insoluble |
Molaritätsrechner
|
In vivo |
|||||
In-vivo-Formulierungsrechner (Klare Lösung)
Schritt 1: Geben Sie die untenstehenden Informationen ein (Empfohlen: Ein zusätzliches Tier zur Berücksichtigung von Verlusten während des Experiments)
Schritt 2: Geben Sie die In-vivo-Formulierung ein (Dies ist nur der Rechner, keine Formulierung. Bitte kontaktieren Sie uns zuerst, wenn es im Abschnitt "Löslichkeit" keine In-vivo-Formulierung gibt.)
Berechnungsergebnisse:
Arbeitskonzentration: mg/ml;
Methode zur Herstellung der DMSO-Stammlösung: mg Wirkstoff vorgelöst in μL DMSO ( Konzentration der Stammlösung mg/mL, Bitte kontaktieren Sie uns zuerst, wenn die Konzentration die DMSO-Löslichkeit der Wirkstoffcharge überschreitet. )
Methode zur Herstellung der In-vivo-Formulierung: Nehmen Sie μL DMSO Stammlösung, dann hinzufügenμL PEG300, mischen und klären, dann hinzufügenμL Tween 80, mischen und klären, dann hinzufügen μL ddH2O, mischen und klären.
Methode zur Herstellung der In-vivo-Formulierung: Nehmen Sie μL DMSO Stammlösung, dann hinzufügen μL Maisöl, mischen und klären.
Hinweis: 1. Bitte stellen Sie sicher, dass die Flüssigkeit klar ist, bevor Sie das nächste Lösungsmittel hinzufügen.
2. Achten Sie darauf, das/die Lösungsmittel der Reihe nach hinzuzufügen. Sie müssen sicherstellen, dass die bei der vorherigen Zugabe erhaltene Lösung eine klare Lösung ist, bevor Sie mit der Zugabe des nächsten Lösungsmittels fortfahren. Physikalische Methoden wie Vortex, Ultraschall oder ein heißes Wasserbad können zur Unterstützung des Lösens verwendet werden.
Wirkmechanismus
| Targets/IC50/Ki |
ATPase (HCT 116)
0.2 μM
|
|---|---|
| In vitro |
Brefeldin A (BFA) ist ein Pilzmetabolit, der den Vorwärtstransport zwischen dem endoplasmatischen Retikulum und dem Golgi-Apparat blockiert, was zu einer gestörten Verteilung der Membranproteine führt. Wenn menschliche HCT 116 Kolonkarzinomzellen mit dieser Verbindung behandelt werden, werden morphologische Veränderungen beobachtet, die auf eine Zelldifferenzierung hinweisen. Es übt seine zytotoxischen Wirkungen hauptsächlich durch Induktion von Differenzierung und Apoptose in Tumorzellen aus. Die Behandlung der Streifen mit 20 μg/mL BFA für 6 Stunden hebt die durch Bradykinin induzierte Relaxation in Anwesenheit von 10mM Indomethacin und 30 μM L-NOARG vollständig auf. Die Behandlung mit 20 μg/mL dieser Verbindung hebt die durch Bradykinin induzierten Abnahmen von [Ca2+]i und Spannung im Konzentrationsbereich zwischen 1 nM und 1 mM im Wesentlichen auf. Es hat keinen Einfluss auf die durch Bradykinin oder Substanz P induzierte [Ca2+]i-Erhöhung in Endothelzellen. Die Zugabe des Pilzmetaboliten beeinflusst die spontane Phospholipid-abhängige GTPS-Bindung an myr-rARF1 nicht, hebt aber den retinalen isotonischen Extrakt (RIE)-katalysierten Austausch vollständig auf, mit einer halbmaximalen Hemmung bei 2 μM. Es verhindert eine Vielzahl von Membrantransportwegen und hemmt eine ADP-Ribosylierungsfaktor-spezifische Guanin-Nukleotid-Austauschaktivität, die in Golgi-Membranen oder im Gehirnzytosol vorhanden ist. Die vollständige Verhinderung durch diese Verbindung legt stark nahe, dass der retinale Extrakt einen ARF-spezifischen Guanin-Nukleotid-Austauschfaktor enthält. Die durch retinalen isotonischen Extrakt (RIE) katalysierte GTPS-Freisetzung aus beiden ADP-Ribosylierungsfaktoren (ARFs) wird durch BFA selbst bei 300 μM nur teilweise gehemmt. Es induziert die Fusion des Golgi-Apparats mit dem ER und hebt die hemmende Wirkung des CERT-Inhibitors HPA-12 auf. Die Behandlung mit dieser Verbindung, die die Fusion des Golgi-Apparats und des ER induziert, rettet die Limonoid-induzierte Verhinderung der Sphingomyelin-Biosynthese. Seine Behandlung von CHO-Zellen führt zu einem 2- bis 3-fachen Anstieg der Sphingomyelin-Synthese. Abgesehen von B-CLL-Zellen verursacht BFA Berichten zufolge Apoptose in multiplem Myelom (U266, NCI-H929), Jurkat, HeLa, Leukämie (HL60, K562, BJAB), Dickdarm (HT-29) und Prostata sowie adenozytischen Sarkomzellen. Die Verabreichung von 25 ng/mL dieser Verbindung blockiert das Wachstum von HF4.9- und HF28RA-Zellen vollständig, während höhere Dosen (75 ng/mL) erforderlich sind, um den gleichen Effekt in HF1A3-Zellen zu erzielen. Die Zellproliferation wird innerhalb von 24 Stunden dosisabhängig gehemmt, und je nach Zelllinie wird eine nahezu vollständige Einstellung der 3H-Thymidin-Inkorporation bei 50-75 ng/mL beobachtet (26%, 76%, 87% Hemmung bei 50 ng/ml und 75%, 87%, 92% Hemmung bei 75 ng/mL für HF1A3-, HF4.9- bzw. HF28RA-Zellen). BFA-induziertes Zellsterben erfolgt dosisabhängig unter Verwendung des YO-PRO 1/PI-Assays. Es könnte die HDR (Homology-Directed Repair) Effizienz verbessern und ist ein Verstärker der CRISPR-mediated HDR. |
| In vivo |
Brefeldin A (BFA), ein Lacton-Antibiotikum und spezifischer Inhibitor des Proteintransports, blockiert den Transport von sezernierten und Membranproteinen vom endoplasmatischen Retikulum zum Golgi-Apparat. |
Literatur |
|
Anwendungen
| Methoden | Biomarker | Bilder | PMID |
|---|---|---|---|
| Western blot | p53 / GRP78 |
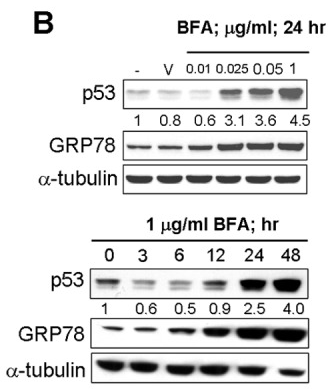
|
22859938 |
| Immunofluorescence | MTP / GBF1 ErbB3 / Calnexin FMNL1 / GM130 |
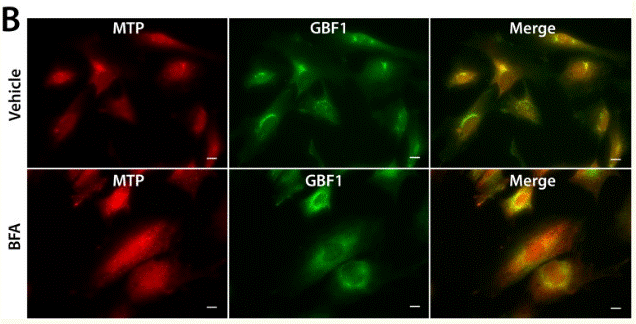
|
26267806 |
| Growth inhibition assay | Cell viability |
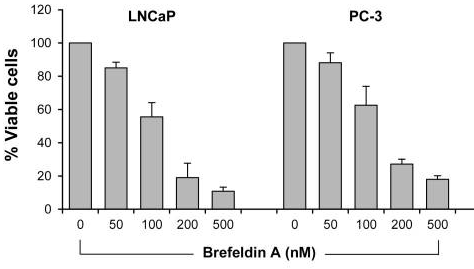
|
28462831 |
Technischer Support
Tel: +1-832-582-8158 Ext:3
Wenn Sie weitere Fragen haben, hinterlassen Sie bitte eine Nachricht.
