nur für Forschungszwecke
Triciribine (API-2) Akt Inhibitor
Kat.-Nr.S1117
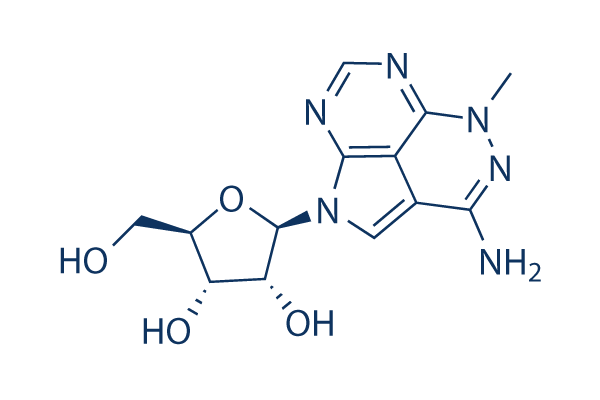
Chemische Struktur
Molekulargewicht: 320.3
Qualitätskontrolle
| Verwandte Ziele | PI3K mTOR GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Weitere Akt Inhibitoren | SC79 AZD5363 (Capivasertib) MK-2206 Dihydrochloride Ipatasertib (GDC-0068) Perifosine GSK690693 Afuresertib (GSK2110183) CCT128930 A-674563 HCl Akti-1/2 |
Zellkultur, Behandlung & Arbeitskonzentration
| Zelllinien | Assay-Typ | Konzentration | Inkubationszeit | Formulierung | Aktivitätsbeschreibung | PMID |
|---|---|---|---|---|---|---|
| L1210 | Function assay | Tested in vitro for cytotoxicity against murine L1210 leukemic cells, IC50=0.035μM | 10882371 | |||
| HFF | Function assay | HCMV plaque assay was performed using HFF cells and effect was calculated as a percentage of reduction in number of plaques, IC50=2.5μM | 10882371 | |||
| BSC-1 | Antiviral assay | Antiviral activity was tested using an enzyme-linked immunosorbent assay (ELISA) to detect HSV-1 (herpes simplex virus type 1) using BSC-1 cells, IC50=23μM | 10882371 | |||
| HFF | Antiviral assay | Antiviral activity against HCMV was determined by plaque reduction assay using HFF cells, IC50=2.5μM | 10882373 | |||
| AA2 | Function assay | 5 hrs | Intracellular phosphorylation (100 uM) in uninfected AA2 cells was studied after 5 hrs of Incubation., Concentration=9μM | 10882373 | ||
| BSC-1 | Antiviral assay | Antiviral activity against HSV-1 was determined using BSC-1 cells by an enzyme-linked immunosorbent assay (ELISA), IC50=23μM | 10882373 | |||
| Huh-7 | Function assay | Compound was tested for its ability to inhibit hepatitis C viral RNA replication in Huh-7 cells (human hepatoma cells), EC50=2μM | 15177464 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells in presence of 20 uM chloroquine by SRB assay, GI50=0.05μM | 18691894 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells in presence of 10 uM chloroquine by SRB assay, GI50=0.56μM | 18691894 | |||
| MDA-MB-231 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-231 cells in presence of 20 uM chloroquine by SRB assay, GI50=0.69μM | 18691894 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by SRB assay, GI50=3.64μM | 18691894 | |||
| MDA-MB-468 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-468 cells in presence of 20 uM chloroquine by SRB assay, GI50=10.29μM | 18691894 | |||
| 184B5 | Cytotoxicity assay | 20 uM | Cytotoxicity against human 184B5 cells at 20 uM chloroquine by SRB assay, GI50=16.96μM | 18691894 | ||
| MDA-MB-468 | Cytotoxicity assay | 10 uM | Cytotoxicity against human MDA-MB-468 cells in presence of 10 uM chloroquine by SRB assay, GI50=20.45μM | 18691894 | ||
| 184B5 | Cytotoxicity assay | 10 uM | Cytotoxicity against human 184B5 cells at 10 uM chloroquine by SRB assay, GI50=34μM | 18691894 | ||
| MDA-MB-231 | Cytotoxicity assay | 10 uM | Cytotoxicity against human MDA-MB-231 cells in presence of 10 uM chloroquine by SRB assay, GI50=37μM | 18691894 | ||
| 184B5 | Cytotoxicity assay | Cytotoxicity against human 184B5 cells by SRB assay, GI50=40μM | 18691894 | |||
| MDA-MB-468 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-468 cells by SRB assay, GI50=43.53μM | 18691894 | |||
| MDM | Antiviral assay | 6 days | Antiviral activity against Human immunodeficiency virus 1 ADA infected in human MDM cells assessed as expression of p24 antigen after 6 days postinfection by ELISA, IC50=0.006μM | 20086149 | ||
| ACH2 | Cytotoxicity assay | Cytotoxicity against human ACH2 cells infected with latent Human immunodeficiency virus 1 by MTS assay, CC50=0.01μM | 20086149 | |||
| ACH2 | Cytotoxicity assay | Cytotoxicity against human ACH2 cells infected with latent Human immunodeficiency virus 1 by MTS assay in presence of tumor necrosis factor alpha, CC50=0.01μM | 20086149 | |||
| CEM-SS | Antiviral assay | 6 days | Antiviral activity against Human immunodeficiency virus 1 3B infected in human CEM-SS cells infected with 0.78 uL of virus stock assessed as expression of p24 antigen after 6 days postinfection by ELISA, IC50<0.01μM | 20086149 | ||
| MDM | Antiviral assay | 6 days | Antiviral activity against Human immunodeficiency virus 1 BAL infected in human MDM cells assessed as expression of p24 antigen after 6 days postinfection by ELISA, IC50=0.018μM | 20086149 | ||
| H9 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 SK1 infected in human H9 cells assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50=0.036μM | 20086149 | |||
| CEM-SS | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B expressing nef protein infecting in CEM-SS cells assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50=0.04μM | 20086149 | |||
| CEM-SS | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B infected in human CEM-SS cells infected with 0.78 uL of virus stock assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50=0.08μM | 20086149 | |||
| H9 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B infected in human H9 cells infected with 25 uL of virus stock assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50<0.1μM | 20086149 | |||
| H9 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B infected in human H9 cells infected with 12.5 uL of virus stock assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50<0.1μM | 20086149 | |||
| H9 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B infected in human H9 cells infected with 6.25 uL of virus stock assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50<0.1μM | 20086149 | |||
| H9 | Antiviral assay | 6 days | Antiviral activity against Human immunodeficiency virus 1 3B infected in human H9 cells infected with 12.5 uL of virus stock assessed as expression of p24 antigen after 6 days postinfection by ELISA, IC50<0.1μM | 20086149 | ||
| H9 | Antiviral assay | 6 days | Antiviral activity against Human immunodeficiency virus 1 3B infected in human H9 cells infected with 6.25 uL of virus stock assessed as expression of p24 antigen after 6 days postinfection by ELISA, IC50<0.1μM | 20086149 | ||
| CEM-SS | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 SK1 infected in human CEM-SS cells assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50=0.13μM | 20086149 | |||
| ACH2 | Antiviral assay | 3 days | Antiviral activity against 5 x 10'3 cells/well Human immunodeficiency virus 1 infected in human ACH2 cells assessed as inhibition of viral Reverse transcriptase after 3 days by [3H]TTP incorporation assay in presence of 5 ng/ml tumor necrosis factor alpha, IC50=0.15μM | 20086149 | ||
| AA5 | Antiviral assay | Antiviral activity against of Human immunodeficiency virus 1 3B infected in AA5 cells infected with 3.13 uL of virus stock assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50<0.17μM | 20086149 | |||
| AA5 | Antiviral assay | Antiviral activity against of Human immunodeficiency virus 1 3B infected in AA5 cells infected with 1.56 uL of virus stock assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50<0.17μM | 20086149 | |||
| AA5 | Antiviral assay | Antiviral activity against of Human immunodeficiency virus 1 3B infected in AA5 cells infected with 0.78 uL of virus stock assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50<0.17μM | 20086149 | |||
| CEM-SS | Antiviral assay | 6 days | Antiviral activity against Human immunodeficiency virus 1 3B infected in human CEM-SS cells infected with 1.56 uL of virus stock assessed as expression of p24 antigen after 6 days postinfection by ELISA, IC50=0.19μM | 20086149 | ||
| CEM-SS | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 harboring plasmid NL4-3 infected in CEM-SS cells assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50=0.2μM | 20086149 | |||
| U1 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 infected in human U1 cells assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50=0.23μM | 20086149 | |||
| CEM-SS | Antiviral assay | 6 days | Antiviral activity against Human immunodeficiency virus 1 3B infected in human CEM-SS cells infected with 3.13 uL of virus stock assessed as expression of p24 antigen after 6 days postinfection by ELISA, IC50=0.24μM | 20086149 | ||
| U1 | Cytotoxicity assay | Cytotoxicity against human U1 cells infected with latent Human immunodeficiency virus 1 by MTS assay, CC50=0.28μM | 20086149 | |||
| U1 | Cytotoxicity assay | Cytotoxicity against human U1 cells infected with latent Human immunodeficiency virus 1 by MTS assay in presence of tumor necrosis factor alpha, CC50=0.28μM | 20086149 | |||
| CEM-SS | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B infected in human CEM-SS cells infected with 1.56 uL of virus stock assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50=0.29μM | 20086149 | |||
| CEM-SS | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B infected in human CEM-SS cells infected with 3.13 uL of virus stock assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50=0.3μM | 20086149 | |||
| CEM-SS | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 D1 harboring Tyr127His mutation in nef protein infecting in CEM-SS cells assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50=0.33μM | 20086149 | |||
| CEM-SS | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 A7 harboring Tyr127His mutation in nef protein infecting in CEM-SS cells assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50=0.5μM | 20086149 | |||
| MDM | Cytotoxicity assay | Cytotoxicity against human MDM cells infected with Human immunodeficiency virus 1 BAL by MTS assay, TC50=0.66μM | 20086149 | |||
| CEM-SS | Antiviral assay | Antiviral activity against Human immunodeficiency virus 2 ROD infected in human CEM-SS cells assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50=1.4μM | 20086149 | |||
| H9 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B infected in human H9 cells infected with 50 uL of virus stock assessed as inhibition of viral Reverse transcriptase by [3H]TTP incorporation assay, IC50=1.53μM | 20086149 | |||
| H9 | Cytotoxicity assay | Cytotoxicity against human H9 cells by MTS assay, IC50=16.3μM | 20086149 | |||
| H9 | Cytotoxicity assay | Cytotoxicity against human H9 cells by MTS assay, CC50=19.6μM | 20086149 | |||
| Klicken Sie hier, um weitere experimentelle Daten zu Zelllinien anzuzeigen | ||||||
Chemische Informationen, Lagerung & Stabilität
| Molekulargewicht | 320.3 | Formel | C13H16N6O4 |
Lagerung (Ab dem Eingangsdatum) | |
|---|---|---|---|---|---|
| CAS-Nr. | 35943-35-2 | SDF herunterladen | Lagerung von Stammlösungen |
|
|
| Synonyme | NSC 154020, VD-0002, vqd-002, TCN | Smiles | CN1C2=NC=NC3=C2C(=CN3C4C(C(C(O4)CO)O)O)C(=N1)N | ||
Löslichkeit
|
In vitro |
DMSO
: 64 mg/mL
(199.81 mM)
Ethanol : 16 mg/mL Water : Insoluble |
Molaritätsrechner
|
In vivo |
|||||
In-vivo-Formulierungsrechner (Klare Lösung)
Schritt 1: Geben Sie die untenstehenden Informationen ein (Empfohlen: Ein zusätzliches Tier zur Berücksichtigung von Verlusten während des Experiments)
Schritt 2: Geben Sie die In-vivo-Formulierung ein (Dies ist nur der Rechner, keine Formulierung. Bitte kontaktieren Sie uns zuerst, wenn es im Abschnitt "Löslichkeit" keine In-vivo-Formulierung gibt.)
Berechnungsergebnisse:
Arbeitskonzentration: mg/ml;
Methode zur Herstellung der DMSO-Stammlösung: mg Wirkstoff vorgelöst in μL DMSO ( Konzentration der Stammlösung mg/mL, Bitte kontaktieren Sie uns zuerst, wenn die Konzentration die DMSO-Löslichkeit der Wirkstoffcharge überschreitet. )
Methode zur Herstellung der In-vivo-Formulierung: Nehmen Sie μL DMSO Stammlösung, dann hinzufügenμL PEG300, mischen und klären, dann hinzufügenμL Tween 80, mischen und klären, dann hinzufügen μL ddH2O, mischen und klären.
Methode zur Herstellung der In-vivo-Formulierung: Nehmen Sie μL DMSO Stammlösung, dann hinzufügen μL Maisöl, mischen und klären.
Hinweis: 1. Bitte stellen Sie sicher, dass die Flüssigkeit klar ist, bevor Sie das nächste Lösungsmittel hinzufügen.
2. Achten Sie darauf, das/die Lösungsmittel der Reihe nach hinzuzufügen. Sie müssen sicherstellen, dass die bei der vorherigen Zugabe erhaltene Lösung eine klare Lösung ist, bevor Sie mit der Zugabe des nächsten Lösungsmittels fortfahren. Physikalische Methoden wie Vortex, Ultraschall oder ein heißes Wasserbad können zur Unterstützung des Lösens verwendet werden.
Wirkmechanismus
| Targets/IC50/Ki |
HIV-1
(CEM-SS, H9, H9IIIB, U1 cells) 20 nM
Akt
(PC3 cells) 130 nM
|
|---|---|
| In vitro |
Triciribine (API-2) zeigt eine maximale Wachstumshemmung bei etwa 1-10 μM und hemmt die Phosphorylierung von Akt sowie von nachgeschaltetem p70S6K bis auf Basalniveaus bei 100μM (IC50 = 130 nM). Es zeigt besonderes Potenzial zur Hemmung des Wachstums in Nf1- und Trp53-mutanten Astrozytomzellen in einer gradabhängigen Weise. Die WHO II K1861-10 Linie wird unvollständig (69% maximale Hemmung) mit einem GI50-Wert von 1,7 μM für diese Verbindung gehemmt, während höhergradige Tumorlinien (KR158, KR130 und SF295) in größerem Maße (>80% maximale Hemmung) bei niedrigeren GI50-Werten (0,4–1,1 mM) gehemmt werden. Wichtig ist, dass es viel weniger wirksam bei der Hemmung primärer Astrozyten ist (GI5013,6 mM), was darauf hindeutet, dass dieser Inhibitor Spezifität für Tumorzellen zeigen könnte. Es hemmt HIV-1 mit einer IC50 von 20 nM. Eine Hemmung von mehr als 90% wird bei 0,1μM erreicht, und eine vollständige Hemmung der Synzytienbildung wird bei 5μM erreicht. Die assoziierte Zelltoxizität in derselben Zelllinie für Triciribine beträgt 46 μM, was zu Selektivitätsindizes von 2250 führt. Es hemmt deutlich die HIV-1-induzierte p24-Core-Antigenproduktion, Reverse Transkriptase und infektiöse Virusproduktion in einer dosisabhängigen Weise unter Verwendung von HIV-1-akut infizierten CEM-SS-, H9- und persistent infizierten H9IIIB- und U1-Zellen. Diese Verbindung hemmt die Akt-Phosphorylierung an Thr308 und Ser473 und die Akt-Aktivität in der menschlichen Prostatakrebs-Zelllinie PC-3. Sie sensibilisiert PC-3-Zellen für TRAIL- und Anti-CD95-induzierte Apoptose, während die Zellen gegenüber DNA-schädigenden Chemotherapeutika resistent bleiben. Es ist hochselektiv für Akt und hemmt nicht die Aktivierung von Phosphatidylinositol-3-Kinase, Phosphoinositid-abhängiger Kinase-1, Proteinkinase C, Serum- und Glukokortikoid-induzierbarer Kinase, Proteinkinase A, Signaltransduktor und Aktivatoren von Transkription 3, extrazellulär signalregulierter Kinase-1/2 oder c-Jun NH2-terminaler Kinase.
|
| Kinase-Assay |
Akt Phosphorylierungsänderungen Assay
|
|
Zellen werden bis zu einer Konfluenz von 80–90 % kultiviert und für 5–10 Minuten mit 1–10 ng/mL epidermalem Wachstumsfaktor oder Plättchen-abgeleitetem Wachstumsfaktor (PDGF)–AA mit oder ohne 10–20 mM U0126 oder LY-294002 stimuliert. Proteinlysate (5–20 μg) werden durch 12%–15% SDS PAGE getrennt und mittels Western Blot auf Akt, phosphoryliertes Akt (Phospho-Ser 473), MAPK und phosphoryliertes MAPK (p44/42 Phospho-Thr202/Tyr204) Antikörper (1:1000) analysiert.
|
|
| In vivo |
Triciribine (API-2), verabreicht bei 1 mg/kg/Tag i.p., hemmt das Tumorwachstum um 90%, 88% und 80% in Nacktmäusen mit OVCAR3-, OVCAR8- und PANC1-Tumoren, die Akt überexprimieren. Diese Verbindung hat jedoch wenig Einfluss auf das Wachstum von OVCAR5- und COLO357-Zellen.
|
Literatur |
|
Anwendungen
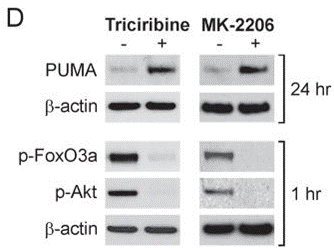
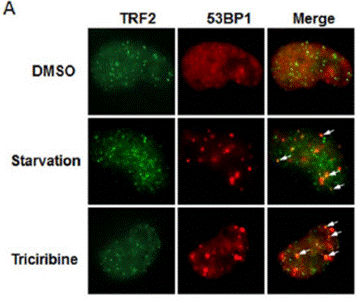
| Methoden | Biomarker | Bilder | PMID |
|---|---|---|---|
| Western blot | PUMA / p-FoxO3a / p-AKT |
|
20978166 |
| Immunofluorescence | TRF2 / 53BP1 |
|
23862686 |
Klinische Studieninformationen
(Daten von https://clinicaltrials.gov, aktualisiert am 2024-05-22)
| NCT-Nummer | Rekrutierung | Erkrankungen | Sponsor/Kooperationspartner | Startdatum | Phasen |
|---|---|---|---|---|---|
| NCT02987127 | Unknown status | Mucosa-Associated Lymphoid Tissue Lymphoma |
National Taiwan University Hospital |
February 2016 | -- |
| NCT00642031 | Completed | Hematologic Malignancies|Leukemia |
Prescient Therapeutics Ltd.|VioQuest Pharmaceuticals |
August 2006 | Phase 1 |
Technischer Support
Tel: +1-832-582-8158 Ext:3
Wenn Sie weitere Fragen haben, hinterlassen Sie bitte eine Nachricht.
