nur für Forschungszwecke
BAY 11-7082 (BAY 11-7821) NF-κB-Inhibitor
Kat.-Nr.S2913
Chemische Struktur
Molekulargewicht: 207.25
Qualitätskontrolle
| Verwandte Ziele | HDAC Antioxidant ROS IκB/IKK Nrf2 AP-1 MALT NOD |
|---|---|
| Weitere NF-κB Inhibitoren | DCZ0415 Omaveloxolone (RTA-408) JSH-23 QNZ (EVP4593) Caffeic Acid Phenethyl Ester SC75741 DHA (Dihydroartemisinin) Withaferin A (WFA) Andrographolide Evodiamine |
Zellkultur, Behandlung & Arbeitskonzentration
| Zelllinien | Assay-Typ | Konzentration | Inkubationszeit | Formulierung | Aktivitätsbeschreibung | PMID |
|---|---|---|---|---|---|---|
| HeLa | Function Assay | 10 μM | 1.5 h | abolishes BPA induced up regulation of FN and MMP-9 | 25797437 | |
| SiHa | Function Assay | 10 μM | 1.5 h | abolishes BPA induced up regulation of FN and MMP-9 | 25797437 | |
| ARPE-19 | Function Assay | 1 μM | 0.5 h | suppresses TG-induced IL-8 promoter activation | 25593029 | |
| HCT116 | Function Assay | 5 μM | 2 h | DMSO | attenuates silymarin-induced downregulation of cyclin D1 | 25479723 |
| HMECs | Function Assay | 5 μM | 2 h | abolishes TNF-α-induced VCAM-1 expression | 25193116 | |
| A549 | Function Assay | 10 µM | 12 h | suppresses Dvl-3 induced activation of p65 | 25156800 | |
| RAW 264.7 | Function Assay | 5 μM | 1 h | inhibits TNF-α and IL-12 p40 production | 25019567 | |
| macrophages | Function Assay | 5 µM | 3 h | partially blocks YPFS-induced expression of iNOS and COX-2 | 24967898 | |
| HUVECs | Function Assay | 3-30 μM | 1 h | reduces the expression of miR-146a in a dose-dependent manner | 24863965 | |
| HeLa | Function Assay | 5 μM | 24 h | DMSO | reduces the activity of TNF-α promoter | 24657783 |
| A549 | Function Assay | 10 μM | 1 h | inhibits the increase of phospho-IκBα in PA103-infected cultures | 24612488 | |
| HUVEC | Function Assay | 20 µM | 0.5 h | DMSO | prevents the induction of EAM expression | 24551209 |
| A549RT-eto | Apoptosis Assay | 10 μM | 24 h | DMSO | accelerates FERO-mediated apoptosis | 24535083 |
| THP-1 | Function Assay | 0.1/1 μM | 0.5 h | abrogates TNF-α secretion as well as the increased secretion of IL-6 and IL-1β | 24378536 | |
| SKCXCR2 | Growth Inhibition Assay | 2 µM | 48 h | decreases cell proliferation significantly | 24376747 | |
| SKCXCR2 | Function Assay | 2 µM | 48 h | blocks the CXCL1-induced cell invasion | 24376747 | |
| OVCXCR2 | Function Assay | 2 µM | 48 h | blocks the CXCL1-induced cell invasion | 24376747 | |
| DSCs | Function Assay | 2.5 μM | 0.5 h | reverses the enhancement of CCL2/CCR2 expression of DSCs induced by IL-33 | 24344240 | |
| WPs | Function Assay | 25 μM | 5 min | suppresses ATP and vWF secretion | 24331207 | |
| A549RT-eto | Apoptosis Assay | 10 μM | 24 h | accelerates F14 extract-mediated apoptosis combined treatment with F14 | 24220725 | |
| A549RT-eto | Function Assay | 10 μM | 24 h | decreases the expression levels of NF-κB and P-gp | 24220725 | |
| FaDu | Function Assay | 2 h | inhibits p65 expression and blocks TNFα-induced TWIST expression | 24220622 | ||
| IVD | Function Assay | 10 μM | 3 d | reverses TNF-α–mediated suppression of the disc matrix macromolecules aggrecan and collagen II | 24176808 | |
| IVD | Function Assay | 10 μM | 3 d | abrogates TNF-α–induced up-regulation of ADAMTS-4 and ADAMTS-5 | 24176808 | |
| iNKT | Function Assay | 10/100 μM | 0.5 h | inhibits the induction of A2AR mRNA and other factor | 24124453 | |
| PC-3 | Function Assay | 2.5/5/10 μM | 0.5 h | blocks IGF-II-induced STS mRNA expression | 24055520 | |
| THP-1 | Function Assay | 10 μM | 1 h | abolishes the effect of rHSP27 on SR-A mRNA | 23939398 | |
| A549 | Function Assay | 1 μM | 48 h | enhances the up-regulation of IκB and subsequent decrease in Bax expression induced by combined stimulation | 23900080 | |
| A549 | Apoptosis Assay | 1 μM | 48 h | reduces the cell death induced by combined stimulation | 23900080 | |
| NCI-N87 | Growth Inhibition Assay | 10/20/30 μM | 6/24 h | suppresses cell viability significantly | 23846545 | |
| AGS | Growth Inhibition Assay | 10/20/30 μM | 6/24 h | suppresses cell viability significantly | 23846545 | |
| MGC80-3 | Growth Inhibition Assay | 10/20/30 μM | 6/24 h | suppresses cell viability significantly | 23846545 | |
| HGC-27 | Function Assay | 7.5/15/30 μM | 6 h | induces the dephosphorylation and up-regulation of IκBα | 23846545 | |
| MGC80-3 | Function Assay | 7.5/15/30 μM | 6 h | induces the dephosphorylation and up-regulation of IκBα | 23846545 | |
| HGC-27 | Apoptosis Assay | 7.5/15/30 μM | 6 h | induces apoptosis in a time- and dose-dependent manner | 23846545 | |
| HBE | Function Assay | 10μM | 3h | abolishes the increases of IL-6 expression induced by CSE | 23824089 | |
| HepG2 | Function Assay | 0.3/1/3 μM | 48 h | reduces IL6-induced PON1 expression | 23791833 | |
| THP-1 | Function Assay | 5 µM | 1 h | DMSO | inhibits MTB-induced NFκB activation | 23634218 |
| THP-1 | Growth Inhibition Assay | 5 µM | 4/8 d | DMSO | reduces the viability of intracellular MTB | 23634218 |
| MDM | Growth Inhibition Assay | 5 µM | 4/8 d | DMSO | reduces the viability of intracellular MTB | 23634218 |
| AM | Growth Inhibition Assay | 5 µM | 4/8 d | DMSO | reduces the viability of intracellular MTB | 23634218 |
| RAW 264 | Function Assay | 0.2-5 µM | 30/60/90 min | inhibits the phosphatase activity of PTP1B | 23578302 | |
| HUVEC | Function Assay | 10 μM | 0.5 h | DMSO | counteractes the loss of Tie2 mRNA | 23563632 |
| HT29 | Function Assay | 10/30/100 μM | 1 h | inhibites both TWEAK-induced p100 processing | 23527154 | |
| HT29 | Function Assay | 10/30/100 μM | 1 h | inhibits TNF-induced phosphorylation and degradation of IκBα | 23527154 | |
| MM.1S | Apoptosis Assay | 30 µM | 3 h | induces MM cell death involves necrosis | 23527154 | |
| KMS-12-BM | Apoptosis Assay | 30 µM | 3 h | induces MM cell death involves necrosis | 23527154 | |
| BAFs | Function Assay | 0.5/1 μM | 24 h | inhibits TNFα/DEX induced CYP19A1 transcripts | 23485457 | |
| SP6.5 | Function Assay | 5 μM | 2 h | decreases translocation of p65 in the nucleus | 23443086 | |
| VUP | Function Assay | 5 μM | 2 h | decreases translocation of p65 in the nucleus | 23443086 | |
| OCM1 | Function Assay | 5 μM | 2 h | decreases translocation of p65 in the nucleus | 23443086 | |
| OM431 | Function Assay | 5 μM | 2 h | decreases translocation of p65 in the nucleus | 23443086 | |
| SP6.5 | Growth Inhibition Assay | 2.5-20 μM | 24 h | IC50=5 μM, exhibits strong anti-proliferative effects in a dose-dependent manner | 23443086 | |
| VUP | Growth Inhibition Assay | 2.5-20 μM | 24 h | IC50=5 μM, exhibits strong anti-proliferative effects in a dose-dependent manner | 23443086 | |
| OCM1 | Growth Inhibition Assay | 2.5-20 μM | 24 h | IC50=5 μM, exhibits strong anti-proliferative effects in a dose-dependent manner | 23443086 | |
| OM431 | Growth Inhibition Assay | 2.5-20 μM | 24 h | IC50=5 μM, exhibits strong anti-proliferative effects in a dose-dependent manner | 23443086 | |
| SP6.5 | Apoptosis Assay | 5 μM | 24 h | induces apoptosis | 23443086 | |
| VUP | Apoptosis Assay | 5 μM | 24 h | induces apoptosis | 23443086 | |
| OCM1 | Apoptosis Assay | 5 μM | 24 h | induces apoptosis | 23443086 | |
| OM431 | Apoptosis Assay | 5 μM | 24 h | induces apoptosis | 23443086 | |
| SP6.5 | Function Assay | 5 μM | 12 h | reduces the migration | 23443086 | |
| VUP | Function Assay | 5 μM | 12 h | reduces the migration | 23443086 | |
| OCM1 | Function Assay | 5 μM | 12 h | reduces the migration | 23443086 | |
| OM431 | Function Assay | 5 μM | 12 h | reduces the migration | 23443086 | |
| HBL-1 | Growth Inhibition Assay | 3 μM | 24/48/72 h | DMSO | slows cell growth modestly | 23441730 |
| RAW 264.7 | Function Assay | 2-15 μM | 1 h | DMSO | suppresses the activation of IKK family members | 23441730 |
| IL-1R | Function Assay | 2-15 μM | 1 h | DMSO | suppresses the activation of IKK family members | 23441730 |
| RAW 264.7 | Function Assay | 15 μM | 1 h | DMSO | suppresses the activation of and JNK | 23441730 |
| IL-1R | Function Assay | 15 μM | 1 h | DMSO | suppresses the activation of and JNK | 23441730 |
| U2OS | Function Assay | 15 μM | 1 h | DMSO | prevents the LPS- or IL-1-stimulated formation of K63-pUb chains | 23441730 |
| MT‐1 | Function Assay | 8 µm | 3 h | decreases the levels of p‐STAT3 and p‐4EBP1 | 23278479 | |
| MT‐2 | Function Assay | 8 µm | 3 h | decreases the levels of p‐STAT3 and p‐4EBP1 | 23278479 | |
| MT‐1 | Function Assay | 8 µm | 3 h | decreases the levels of the p65 subunit of NF‐κB | 23278479 | |
| MT‐2 | Function Assay | 8 µm | 3 h | decreases the levels of the p65 subunit of NF‐κB | 23278479 | |
| MCF-7 | Function Assay | 2.5-15 μM | 0.5 h | DMSO | causes the gradual loss of cell adhesion | 23093227 |
| HaCaT | Function Assay | 5.0 μM | 1 h | attenuates the TCOH-induced production of IL-6 | 23041168 | |
| A549 | Function Assay | 1 h | inhibits LTA-induced SP-A mRNA production significantly | 23031213 | ||
| OA chondrocytes | Function Assay | 10 μM | 1 h | blocks the AGE-BSA-induced gene/protein expression of GRP78 or COX-2 (p<0.05) | 22982228 | |
| RAW264.7 | Function Assay | 15 μM | 15-120 min | blocks the production of NO, PGE2, and TNF-α | 22745523 | |
| RAW264.7 | Growth Inhibition Assay | 5-30 μM | 24 h | inhibits cell growth in a dose-dependent manner | 22745523 | |
| HBL6 | Apoptosis Assay | 0.5/5/25 μM | 6/24 h | decreases cell viability and leeads to apoptosis in a dose-dependent manner | 22074820 | |
| HT29 | Function Assay | 1-10 μM | 10 h | increases HO-1 mRNA and protein expression | 21620964 | |
| Ca9–22 | Apoptosis Assay | 10 μM | 1 h | completely inhibits ALA-PDT-induced apoptosis | 21138480 | |
| Ca9–22 | Function Assay | 10 μM | 1 h | completely abrogates the ALA-PDT-induced JNK activation | 21138480 | |
| A-549 | Growth Inhibition Assay | 10 μM | 24/48 h | inhibits cell growth in a time-dependent manner | 20866043 | |
| AP | Function Assay | 5/10 μM | 48 h | downregulates the BAD protein level a dose-dependent manner | 20596645 | |
| AQ1 | Function Assay | 5/10 μM | 48 h | downregulates the BAD protein level a dose-dependent manner | 20596645 | |
| AP | Function Assay | 20 μM | 4/8 h | downregulates the BAD protein level a time-dependent manner | 20596645 | |
| AQ1 | Function Assay | 20 μM | 4/8 h | downregulates the BAD protein level a time-dependent manner | 20596645 | |
| THP-1 | Function Assay | 5 μM | 0.5 h | attenuates the LPS-induced p-IκBα protein by 72% | 20309718 | |
| K562 | Growth Inhibition Assay | 2-30 μM | 24 h | IC50=8 μM,inhibits cell growth in a dose-dependent manner | 19646807 | |
| Jurket | Growth Inhibition Assay | 2-30 μM | 24 h | IC50=7.1 μM, inhibits cell growth in a dose-dependent manner | 19646807 | |
| U937 | Growth Inhibition Assay | 2-30 μM | 24 h | IC50=10.5 μM, inhibits cell growth in a dose-dependent manner | 19646807 | |
| PBMC | Growth Inhibition Assay | 2-30 μM | 24 h | IC50=40.2 μM, inhibits cell growth in a dose-dependent manner | 19646807 | |
| K562 | Apoptosis Assay | 2-20 μM | 24 h | induces a dose-dependent apoptosis | 19646807 | |
| THP1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human THP1 cells assessed as reduction in cell viability after 72 hrs by MTT assay, TC50 = 1.5 μM. | 28410442 | ||
| RAW264.7 | Function assay | 6 hrs | Inhibition of LPS-induced NF-kappaB activation in mouse RAW264.7 cells treated 30 mins before LPS challenge measured after 6 hrs by luciferase reporter gene assay, IC50 = 1.72 μM. | 24315191 | ||
| HEK293 | Function assay | 6 hrs | Inhibition of TNF-alpha-induced NF-kappaB activity in HEK293 cells after 6 hrs by luciferase reporter gene assay, IC50 = 2 μM. | 24533857 | ||
| HEK293 | Function assay | 6 hrs | Inhibition of TNF-alpha-induced NF-kappaB activity in HEK293 cells after 6 hrs by luciferase reporter gene assay, IC50 = 2 μM. | 24992702 | ||
| HEK293 | Function assay | 6 hrs | Inhibition of TNFalpha-induced NF-kappaB activity (unknown origin) transfected in HEK293 cells after 6 hrs by luciferase reporter gene assay, IC50 = 2 μM. | 26343828 | ||
| HEK293 | Function assay | 6 hrs | Inhibition of TNFalpha-induced NF-kappaB activity expressed in human HEK 293 cells after 6 hrs by luciferase reporter gene assay, IC50 = 2 μM. | 22850207 | ||
| HEK293 | Function assay | 6 hrs | Inhibition of TNFalpha-induced human NFkappaB activity in HEK293 cells incubated for 6 hrs followed by compound wash out measured after 5 mins by by luciferase assay, IC50 = 2.01 μM. | 22712432 | ||
| HEK293 | Cytotoxicity assay | Cytotoxicity against HEK293 cells, IC50 = 3.8 μM. | 24533857 | |||
| HEK293 | Function assay | 6 hrs | Inhibition of TNFalpha-induced NFkappaB (unknown origin) activation expressed in HEK293 cells after 6 hrs by luciferase reporter gene assay, IC50 = 5 μM. | 23316950 | ||
| HEK293 | Function assay | Effect on Cdc2 expressed in HEK293 cells assessed as effect on Cdc2:Cdc25C interaction complexes in presence of camptothecin by EYFP and/or YFP Venus fragment based reporter gene assay | 16680159 | |||
| HEK293 | Function assay | 20 uM | 24 hrs | Inhibition of TNF-alpha stimulated NFkappaB transactivation in HEK293 cells at 20 uM measured after 24 hrs by dual luciferase reporter gene assay | 27736063 | |
| RAW264.7 | Function assay | 20 uM | 1 hr | Inhibition of LPS-induced NFkB activation in mouse RAW264.7 cells assessed as reduction in nuclear translocation of p65 at 20 uM preincubated for 1 hr followed by LPS stimulation measured after 3 hrs by Western blot method | 28667873 | |
| RAW264.7 | Function assay | 20 uM | 6 hrs | Inhibition of LPS-induced NF-kappaB activation in mouse RAW264.7 cells at 20 uM treated 30 mins before LPS challenge measured after 6 hrs by luciferase reporter gene assay | 24315191 | |
| RAW264.7 | Antinflammatory assay | 20 uM | 18 hrs | Antinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced nitric oxide production at 20 uM treated 30 mins before LPS challenge measured after 18 hrs by Griess assay | 24315191 | |
| THP1 | Antinflammatory assay | 5 uM | 24 hrs | Antiinflammatory activity in human THP1 cells assessed as inhibition of TPA/ionomycin-induced extracellular IL-1beta level at 5 uM incubated 1 hr prior to TPA/ionomycin challenge measured after 24 hrs by ELISA | 24400858 | |
| THP1 | Antinflammatory assay | 5 uM | 24 hrs | Antiinflammatory activity in human THP1 cells assessed as inhibition of TPA/ionomycin-induced extracellular TNF-alpha production at 5 uM incubated 1 hr prior to TPA/ionomycin challenge measured after 24 hrs by ELISA | 24400858 | |
| THP1 | Antinflammatory assay | 5 uM | 24 hrs | Antiinflammatory activity in human THP1 cells assessed as inhibition of TPA/ionomycin-induced intracellular proIL-1beta level at 5 uM incubated 1 hr prior to TPA/ionomycin challenge measured after 24 hrs by ELISA | 24400858 | |
| THP1 | Antinflammatory assay | 5 uM | 24 hrs | Antiinflammatory activity in human THP1 cells assessed as inhibition of TPA/ionomycin-induced intracellular IL-1beta level at 5 uM incubated 1 hr prior to TPA/ionomycin challenge measured after 24 hrs by ELISA | 24400858 | |
| RAW264.7 | Function assay | 0.3 ug/ml | 12 hrs | Inhibition of LPS-induced NF-kB p65 phosphorylation in mouse RAW264.7 cells at 0.3 ug/ml preincubated for 12 hrs followed by LPS stimulation for 3 hrs by Western blot method | 28284806 | |
| RAW264.7 | Function assay | 0.3 ug/ml | 12 hrs | Inhibition of LPS-induced NF-kB p65 activation in mouse RAW264.7 cells at 0.3 ug/ml preincubated for 12 hrs followed by LPS stimulation for 3 hrs by DAPI staining based inverted fluorescence microscopic method | 28284806 | |
| RAW264.7 | Function assay | 0.3 ug/ml | 12 hrs | Inhibition of NF-kB p65 in mouse RAW264.7 cells assessed as reduction in LPS-induced iNOS expression at 0.3 ug/ml preincubated for 12 hrs followed by LPS stimulation for 3 hrs by Western blot method | 28284806 | |
| RAW264.7 | Function assay | 0.3 ug/ml | 12 hrs | Inhibition of NF-kB p65 in mouse RAW264.7 cells assessed as reduction in LPS-induced COX2 expression at 0.3 ug/ml preincubated for 12 hrs followed by LPS stimulation for 3 hrs by Western blot method | 28284806 | |
| HEK293 | Function assay | 20 uM | 24 hrs | Inhibition of TNFalpha-induced NFkappaB activation in HEK293 cells at 20 uM after 24 hrs by dual luciferase reporter gene assay | 28873303 | |
| RAW264.7 | Function assay | 20 uM | 2 hrs | Inhibition of NFkappaB nuclear translocation in LPS-stimulated mouse RAW264.7 cells at 20 uM pretreated for 2 hrs followed by LPS-induction by DAPI-staining based immunofluorescence microscopic method | 29759725 | |
| BGC823 | Function assay | 5 uM | 12 hrs | Inhibition of colony formation in human BGC823 cells at 5 uM treated for 12 hrs followed by incubation in drug free medium for 14 days by crystal violet staining based assay | 28881286 | |
| SGC7901 | Function assay | 5 uM | 12 hrs | Inhibition of colony formation in human SGC7901 cells at 5 uM treated for 12 hrs followed by incubation in drug free medium for 14 days by crystal violet staining based assay | 28881286 | |
| RAW264.7 | Function assay | 10 uM | 2 hrs | Inhibition of LPS-induced IL-6 mRNA expression in mouse RAW264.7 cells at 10 uM pre-incubated for 2 hrs before LPS stimulation for 24 hrs by qRT-PCR method | 27038497 | |
| RAW264.7 | Function assay | 10 uM | 2 hrs | Inhibition of LPS-induced IL-1beta mRNA expression in mouse RAW264.7 cells at 10 uM pre-incubated for 2 hrs before LPS stimulation for 24 hrs by qRT-PCR method | 27038497 | |
| RAW264.7 | Function assay | 10 uM | 2 hrs | Inhibition of LPS-induced iNOS mRNA expression in mouse RAW264.7 cells at 10 uM pre-incubated for 2 hrs before LPS stimulation for 24 hrs by qRT-PCR method | 27038497 | |
| Klicken Sie hier, um weitere experimentelle Daten zu Zelllinien anzuzeigen | ||||||
Chemische Informationen, Lagerung & Stabilität
| Molekulargewicht | 207.25 | Formel | C10H9NO2S |
Lagerung (Ab dem Eingangsdatum) | |
|---|---|---|---|---|---|
| CAS-Nr. | 19542-67-7 | SDF herunterladen | Lagerung von Stammlösungen |
|
|
| Synonyme | BAY 11-7821 | Smiles | CC1=CC=C(C=C1)S(=O)(=O)C=CC#N | ||
Löslichkeit
|
In vitro |
DMSO
: 41 mg/mL
(197.82 mM)
Ethanol : 10 mg/mL Water : Insoluble |
Molaritätsrechner
|
In vivo |
|||||
In-vivo-Formulierungsrechner (Klare Lösung)
Schritt 1: Geben Sie die untenstehenden Informationen ein (Empfohlen: Ein zusätzliches Tier zur Berücksichtigung von Verlusten während des Experiments)
Schritt 2: Geben Sie die In-vivo-Formulierung ein (Dies ist nur der Rechner, keine Formulierung. Bitte kontaktieren Sie uns zuerst, wenn es im Abschnitt "Löslichkeit" keine In-vivo-Formulierung gibt.)
Berechnungsergebnisse:
Arbeitskonzentration: mg/ml;
Methode zur Herstellung der DMSO-Stammlösung: mg Wirkstoff vorgelöst in μL DMSO ( Konzentration der Stammlösung mg/mL, Bitte kontaktieren Sie uns zuerst, wenn die Konzentration die DMSO-Löslichkeit der Wirkstoffcharge überschreitet. )
Methode zur Herstellung der In-vivo-Formulierung: Nehmen Sie μL DMSO Stammlösung, dann hinzufügenμL PEG300, mischen und klären, dann hinzufügenμL Tween 80, mischen und klären, dann hinzufügen μL ddH2O, mischen und klären.
Methode zur Herstellung der In-vivo-Formulierung: Nehmen Sie μL DMSO Stammlösung, dann hinzufügen μL Maisöl, mischen und klären.
Hinweis: 1. Bitte stellen Sie sicher, dass die Flüssigkeit klar ist, bevor Sie das nächste Lösungsmittel hinzufügen.
2. Achten Sie darauf, das/die Lösungsmittel der Reihe nach hinzuzufügen. Sie müssen sicherstellen, dass die bei der vorherigen Zugabe erhaltene Lösung eine klare Lösung ist, bevor Sie mit der Zugabe des nächsten Lösungsmittels fortfahren. Physikalische Methoden wie Vortex, Ultraschall oder ein heißes Wasserbad können zur Unterstützung des Lösens verwendet werden.
Wirkmechanismus
| Targets/IC50/Ki |
E2-conjugating enzymes
(Cell-free assay) USP7
(Cell-free assay) 0.19 μM
USP21
(Cell-free assay) 0.96 μM
USP6
(Cell-free assay) 1.7 μM
IκBα phosphorylation
(Tumor cells) 10 μM
|
|---|---|
| In vitro |
BAY 11-7082 hebt die NF-κB-DNA-Bindung vollständig und spezifisch auf, wodurch das NF-κB-induzierbare Zytokin IL-6 herunterreguliert und Apoptose induziert wird. Diese Verbindung (< 8 μM) ist in der Lage, sowohl die basale als auch die TNFα-stimulierte NFκB-Luciferase-Aktivität dosisabhängig wirksam zu hemmen. Sie (8 μM) hemmt die Proliferationsrate in NCI-H1703-Zellen stark. Diese Verbindung (5 μM) reduziert schnell und effizient die DNA-Bindung von NF-kappaB in HTLV-I-infizierten T-Zelllinien und reguliert die Expression des antiapoptotischen Gens Bcl-x(L) herunter, während sie wenig Einfluss auf die DNA-Bindung eines anderen Transkriptionsfaktors, AP-1, hat. Diese chemisch induzierte Apoptose primärer ATL-Zellen ist ausgeprägter als die normaler peripherer Blutmononuklearzellen, und die Apoptose dieser Zellen ist auch mit einer Herunterregulierung der NF-kappaB-Aktivität verbunden. Sie (5 μM) induziert selektiv die Apoptose von HTLV-I-infizierten T-Zelllinien, verbunden mit einer Herunterregulierung der Expression von Cyclin D1, Cyclin D2 und Bcl-xL. Diese Verbindung (100 μM) verhindert die Kern-Translokation von p65, die durch NMDA ausgelöst wird, und den NMDA-induzierten Anstieg der NF-κB-Bindung in Maus-Hippocampus-Schnitten. Sie verhindert die NMDA-Toxizität in der CA1-Region von Hippocampus-Schnitten mit 40% Neuroprotektion bei 20 μM und 70% Neuroprotektion bei 100 μM. Diese Chemikalie hemmt bei allen getesteten Konzentrationen signifikant die NF-κB p65 DNA-Bindungsaktivität im Fettgewebe, während sie im Skelettmuskel bei 50 μM und 100 μM die NF-κB p65 DNA-Bindungsaktivität signifikant hemmt. Sie (100 μM) reduziert das IKK-β-Protein in menschlichem Fettgewebe und Skelettmuskel. Diese Verbindung (100 μM) verringert signifikant die Freisetzung von TNF-α aus dem Fettgewebe, während die Freisetzung von IL-6 und IL-8 bei allen getesteten Konzentrationen dieser Chemikalie signifikant gehemmt wird. Sie (50 μM) verringert signifikant die Freisetzung von TNF-α, IL-6 und IL-8 im Skelettmuskel. Diese Verbindung inaktiviert auch die E2-konjugierenden Enzyme Ubc (Ubiquitin-konjugierende) 13 und UbcH7 sowie die E3-Ligase LUBAC (linearer Ubiquitin-Assemblierungs-Komplex) und induziert somit den Tod von B-Zell-Lymphomen und leukämischen T-Zellen. |
| In vivo |
BAY 11-7082, ein NF-κB-Inhibitor, induziert Apoptose und S-Phasen-Arrest in Magenkrebszellen. |
Literatur |
|
Anwendungen
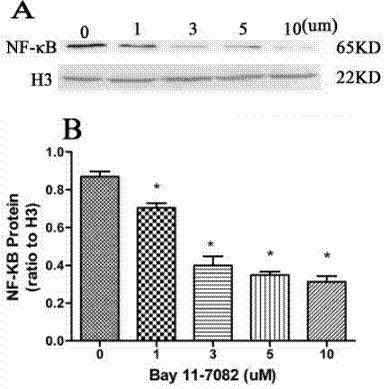
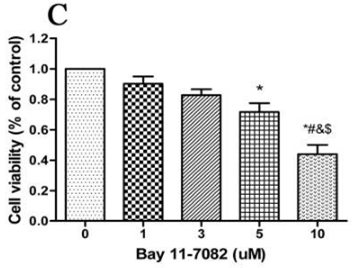
| Methoden | Biomarker | Bilder | PMID |
|---|---|---|---|
| Western blot | NF-κB p-IKKβ/ IκBα p-IRAK4 / IRAK4 NF-κB p-IKKβ/ IκBα p-IRAK4 / IRAK4 |
|
31332209 |
| Growth inhibition assay | Cell viability Cell viability |
|
31332209 |
Technischer Support
Tel: +1-832-582-8158 Ext:3
Wenn Sie weitere Fragen haben, hinterlassen Sie bitte eine Nachricht.
