cAMP inhibitors are a class of bioactive molecules or compounds that specifically interfere with the synthesis, signaling, or downstream effects of cyclic adenosine monophosphate (cAMP), a pivotal second messenger in eukaryotic cellular signaling pathways. The regulation of cAMP levels and its downstream cascades is critical for numerous physiological processes, including cell proliferation, differentiation, metabolism, and neurotransmission, as well as pathological conditions such as cancer, cardiovascular diseases, and neurological disorders. In scientific research, cAMP inhibitors serve as essential tools to dissect the complexity of cAMP-mediated signaling networks, while also holding significant potential for the development of targeted therapeutic agents.
cAMP Inhibitoren (cAMP Inhibitors)
Weitere GPCR & G Protein Inhibitoren
Isoformspezifische Produkte
| Kat.-Nr. | Produktname | Informationen | Publikationen | Validierung |
|---|---|---|---|---|
| S2449 | Forskolin (Colforsin) | Forskolin (Colforsin) ist ein allgegenwärtiger Aktivator der eukaryotischen Adenylylcyclase (AC) in einer Vielzahl von Zelltypen, der üblicherweise zur Erhöhung der cAMP-Spiegel in der Zellphysiologie-Studie und -Forschung verwendet wird. Forskolin aktiviert auch die PXR- und FXR-Aktivität. Forskolin stimuliert die Autophagie. |
|
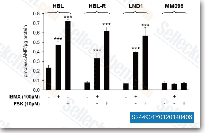
|
| S8415 | PACAP 1-38 | PACAP 1-38 (Pituitary Adenylate Cyclase Activating Polypeptide 38) ist ein hochwirksamer PACAP-Rezeptoragonist (Kd = 100 pM). Er stimuliert die Adenylatcyclase und die Phagozytose. |
|
|
| S8283 | SQ22536 | SQ22536 (9-(Tetrahydrofuran-2-yl)-9h-purin-6-amin) ist ein Inhibitor der Adenylylcyclase mit einem IC50 von 1,4 μM. Diese Verbindung kann PGE1-stimulierte Erhöhungen der cAMP-Spiegel in intakten menschlichen Thrombozyten hemmen. |
|

|
| S7499 | ESI-09 | ESI-09 ist ein spezifischer Austauschprotein, das direkt durch cAMP (EPAC) aktiviert wird Inhibitor mit einer IC50 von 3,2 μM und 1,4 μM für EPAC1 bzw. EPAC2, >100-fache Selektivität gegenüber PKA. |
|
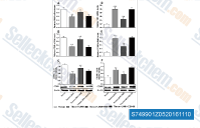
|
| S4552 | Bithionol | Bithionol (Actamer) ist ein potenter Inhibitor der löslichen Adenylylcyclase (sAC) mit einer IC50 von 4 μM und besitzt antibakterielle und anthelmintische Eigenschaften sowie algizide Aktivität. |
|
|
| S8416 | PACAP 6-38 acetate | PACAP 6-38 acetate ist ein nicht stimulierender kompetitiver Antagonist von PACAP (pituitary adenylate cyclase-activating polypeptide) mit einem IC50-Wert von 2 nM. Es wirkt auch als funktioneller CARTp-Antagonist in vivo. |
|
|
| S7500 | HJC0350 | HJC0350 ist ein potenter und selektiver EPAC2-Inhibitor mit einer IC50 von 0,3 μM, der keine Hemmung von Epac1 zeigt. |
|
|
| S3382 | ESI-05 | ESI-05 (NSC 116966) ist ein spezifischer EPAC2 (exchange protein directly activated by cAMP 2) Antagonist mit einer IC50 von 0,4 µM. Diese Verbindung hemmt die cAMP-vermittelte Aktivierung von EPAC2 und die EAPC2-vermittelte Rap1-Aktivierung. | ||
| E6418New | MDL12330A | MDL12330A ist ein Inhibitor der Adenylylcyclase, der den Vasopressin-induzierten Kurzschlussstrom (SCC) blockiert. Er hemmt auch die cAMP-Phosphodiesterase, was seine Wirkung auf die Adenylylcyclase maskieren könnte. | ||
| S8414 | PACAP 1-27 | Pituitary adenylate cyclase activating polypeptide (PACAP 1-27) ist ein potenter PACAP receptor-Antagonist. | ||
Biology Function of cAMP Inhibitors in Cyclic AMP-Mediated Signaling Networks
The biology function of cAMP inhibitors is inherently linked to their ability to modulate the cyclic AMP signaling pathway, which is initiated by the activation of G protein-coupled receptors (GPCRs). Upon ligand binding, GPCRs activate adenylyl cyclases (ACs), which catalyze the conversion of ATP to cAMP. The accumulated cAMP then binds to downstream effectors, such as protein kinase A (PKA), exchange proteins directly activated by cAMP (EPACs), and cyclic nucleotide-gated (CNG) channels, triggering a series of cellular responses. cAMP inhibitors exert their biological functions by targeting different components of this pathway, thereby regulating the amplitude and duration of cAMP signaling.
Regulation of Cell Proliferation and Apoptosis
One of the most well-characterized biology functions of cAMP inhibitors is their role in regulating cell proliferation and apoptosis. Abnormal activation of the cyclic AMP pathway is frequently observed in various cancer types; for example, overexpression of ACs or mutations in PKA subunits can lead to uncontrolled cell growth. cAMP inhibitors that target ACs, such as forskolin analogs with inhibitory activity, can reduce intracellular cAMP levels, thereby inhibiting the proliferation of cancer cells. In contrast, in some normal cell types, appropriate inhibition of cAMP signaling can promote apoptosis, which is crucial for maintaining tissue homeostasis. For instance, in neuronal cells, excessive cAMP accumulation can lead to neurotoxicity, and cAMP inhibitors have been shown to protect neurons by suppressing cAMP-dependent apoptotic pathways. These findings highlight the context-dependent biology functions of cAMP inhibitors in cell fate determination.
Modulation of Metabolic Processes
Another important biology function of cAMP inhibitors is the modulation of metabolic processes, particularly glucose and lipid metabolism. The cyclic AMP pathway plays a central role in regulating insulin secretion and glucose uptake in pancreatic β-cells and adipose tissue. In pancreatic β-cells, increased cAMP levels enhance insulin secretion, and cAMP inhibitors can reduce insulin release by inhibiting AC activity or PKA signaling. This function is relevant to the research of type 2 diabetes, as dysregulated cAMP signaling is associated with impaired insulin secretion. Additionally, in adipose tissue, cAMP promotes lipolysis by activating PKA, which phosphorylates hormone-sensitive lipase. cAMP inhibitors can suppress lipolysis, thereby regulating lipid accumulation and energy balance. These metabolic regulatory functions make cAMP inhibitors valuable tools for studying metabolic diseases.
Mechanism of Action of cAMP Inhibitors Targeting Cyclic AMP Signaling Components
The mechanism of action of cAMP inhibitors is diverse, depending on their specific molecular targets within the cyclic AMP signaling pathway. These inhibitors can be classified into different categories based on the components they target, including inhibitors of ACs, antagonists of cAMP receptors, and modulators of cAMP degradation. Understanding the precise mechanism of action of each type of cAMP inhibitor is essential for their rational application in scientific research and drug development.
Inhibition of Adenylyl Cyclases (ACs)
The primary mechanism of action of many cAMP inhibitors is the direct inhibition of ACs, the enzymes responsible for cyclic AMP synthesis. ACs are a family of membrane-bound or soluble enzymes, with 10 membrane-bound isoforms (AC1-AC10) and one soluble isoform (sAC) identified in humans. Different AC isoforms exhibit tissue-specific expression and distinct regulatory properties, providing potential targets for selective inhibition. For example, the compound SQ22536 is a non-selective AC inhibitor that binds to the catalytic domain of ACs, preventing the conversion of ATP to cAMP. In contrast, more recent research has focused on developing isoform-selective AC inhibitors; for instance, AC5 inhibitors have been shown to target cardiac tissue, where AC5 overexpression is associated with heart failure. The mechanism of action of these isoform-selective inhibitors involves binding to unique allosteric sites on specific AC isoforms, thereby achieving tissue-specific regulation of cyclic AMP levels.
Antagonism of Cyclic AMP Receptors
Another important mechanism of action of cAMP inhibitors is the antagonism of cAMP receptors, such as PKA and EPACs. PKA is a tetrameric enzyme composed of two regulatory subunits and two catalytic subunits; cAMP binding to the regulatory subunits induces a conformational change, releasing the active catalytic subunits, which then phosphorylate downstream substrates. cAMP inhibitors that act as PKA antagonists bind to the cAMP-binding sites on the regulatory subunits, preventing cAMP from activating PKA. For example, the compound H89 is a widely used PKA inhibitor that competes with cAMP for binding to the regulatory subunits, thereby inhibiting PKA-mediated phosphorylation. Similarly, EPACs are guanine nucleotide exchange factors that are activated by cAMP binding; EPAC-specific inhibitors have been developed to block cAMP-EPAC signaling, which is involved in processes such as cell adhesion and migration. The mechanism of action of these receptor antagonists is critical for dissecting the specific roles of PKA and EPAC pathways in cAMP-mediated cellular responses.
Enhancement of Cyclic AMP Degradation
A third mechanism of action of cAMP inhibitors is the enhancement of cAMP degradation by phosphodiesterases (PDE s), which are enzymes that hydrolyze cAMP to 5'-AMP, thereby terminating cAMP signaling. PDEs are a large family of enzymes with different isoforms that exhibit tissue-specific expression and substrate specificity. Some cAMP inhibitors act as PDE activators, increasing the activity of PDEs and thus reducing intracellular cAMP levels. For example, certain natural compounds have been shown to activate PDE4, a isoform that is highly expressed in immune cells, thereby inhibiting cAMP-mediated inflammation. Alternatively, some PDE inhibitors are used in research to increase cAMP levels, but the opposite effect (PDE activation) can also be achieved with specific compounds, representing a distinct mechanism of cAMP inhibition. This mechanism of action is particularly relevant in research focused on the regulation of cAMP signaling termination and the role of PDEs in disease.
Pharmacology of cAMP Inhibitors: From In Vitro Research to In Vivo Applications
The pharmacology of cAMP inhibitors encompasses their absorption, distribution, metabolism, excretion (ADME) properties, as well as their efficacy, selectivity, and toxicity, which are critical for their application in scientific research and potential clinical translation. In research settings, cAMP inhibitors are used in both in vitro and in vivo studies to investigate the role of cyclic AMP signaling in various biological processes and diseases. The pharmacokinetic and pharmacodynamic properties of these inhibitors vary widely depending on their chemical structure, molecular target, and route of administration.
In Vitro Pharmacology of cAMP Inhibitors
In in vitro research, the pharmacology of cAMP inhibitors is primarily evaluated based on their potency, selectivity, and cell permeability. Potency is typically measured by the half-maximal inhibitory concentration (IC50), which reflects the concentration of the inhibitor required to achieve 50% inhibition of its target (e.g., ACs, PKA). Selectivity is a key parameter, as non-selective inhibitors may interfere with other signaling pathways, leading to off-target effects. For example, H89, while widely used as a PKA inhibitor, also exhibits some inhibitory activity against other kinases, which must be considered when interpreting experimental results. Cell permeability is another important pharmacokinetic property, as cAMP inhibitors need to cross the cell membrane to reach their intracellular targets. Small-molecule inhibitors, such as SQ22536 and H89, are generally cell-permeable, making them suitable for in vitro cell culture studies. In addition, in vitro pharmacology studies often involve evaluating the time-dependent effects of cAMP inhibitors, as the duration of inhibition can vary depending on the stability of the compound and the turnover rate of its target.
In Vivo Pharmacology of cAMP Inhibitors
In in vivo research, the pharmacology of cAMP inhibitors is more complex, involving ADME properties and in vivo efficacy. The route of administration (e.g., oral, intravenous, intraperitoneal) significantly affects the absorption and distribution of the inhibitor. For example, oral inhibitors need to be stable in the gastrointestinal tract and efficiently absorbed into the bloodstream, while intravenous inhibitors bypass absorption and directly enter the circulation. Distribution of cAMP inhibitors to target tissues is critical for their efficacy; tissue-specific distribution can be enhanced by modifying the chemical structure of the inhibitor to improve its affinity for specific tissue markers. Metabolism and excretion of cAMP inhibitors are primarily mediated by the liver and kidneys, respectively; metabolic stability is an important factor, as rapidly metabolized inhibitors may require frequent dosing to maintain effective concentrations. Toxicity is another key consideration in in vivo studies; non-specific inhibition of cyclic AMP signaling can lead to adverse effects, such as cardiovascular dysfunction or metabolic disorders. Therefore, the development of selective cAMP inhibitors with reduced toxicity is a major focus of pharmacology research in this field.
In conclusion, cAMP inhibitors are invaluable tools in scientific research, providing insights into the biology function and mechanism of action of cyclic AMP signaling pathways. Their pharmacology properties determine their suitability for different research applications, from in vitro cell studies to in vivo animal models. Future research on cAMP inhibitors will likely focus on the development of more selective and potent compounds, as well as the exploration of their therapeutic potential in diseases associated with dysregulated cAMP signaling. By continuing to unravel the complex interactions between cAMP inhibitors and the cyclic AMP pathway, researchers can advance our understanding of fundamental biological processes and pave the way for novel therapeutic strategies.
