nur für Forschungszwecke
TMZ(Temozolomide) Alkylierungsmittel
Kat.-Nr.S1237
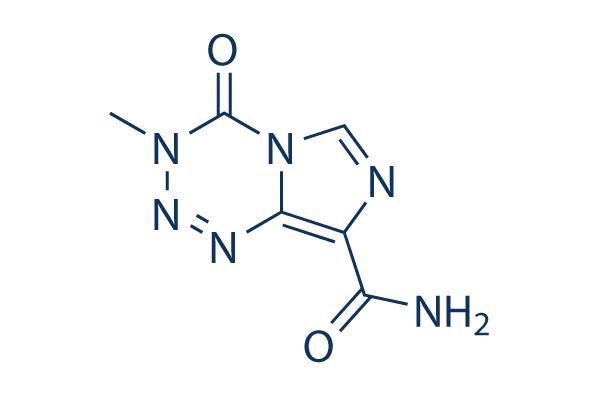
Chemische Struktur
Molekulargewicht: 194.15
Qualitätskontrolle
| Verwandte Ziele | HDAC PARP ATM/ATR DNA-PK WRN Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Weitere DNA/RNA Synthesis Inhibitoren | CX-5461 (Pidnarulex) B02 SCR7 Favipiravir (T-705) EED226 RK-33 BMH-21 Triapine (3-AP) Carmofur YK-4-279 |
Zellkultur, Behandlung & Arbeitskonzentration
| Zelllinien | Assay-Typ | Konzentration | Inkubationszeit | Formulierung | Aktivitätsbeschreibung | PMID |
|---|---|---|---|---|---|---|
| Kelly | Growth Inhibition Assay | 48 h | IC50=139.20 ± 5.95 μM | 25960282 | ||
| KellyCis83 | Growth Inhibition Assay | 48 h | IC50=251.00 ± 15.75 μM | 25960282 | ||
| SK-N-AS | Growth Inhibition Assay | 48 h | IC50=227.70 ± 22.15 μM | 25960282 | ||
| SK-N-ASCis24 | Growth Inhibition Assay | 48 h | IC50=480.60 ± 101.15 μM | 25960282 | ||
| CHP-212 | Growth Inhibition Assay | 48 h | IC50=7.97 ± 0.69 μM | 25960282 | ||
| CHP-212Cis100 | Growth Inhibition Assay | 48 h | IC50=9.55 ± 0.88 μM | 25960282 | ||
| U87 | Function Assay | 100 μM | 24-72 h | induces DcR1 expression | 25808868 | |
| LN229 | Growth Inhibition Assay | 0-50 μM | IC50=16 μM | 25750273 | ||
| TR-LN229 | Growth Inhibition Assay | 0-50 μM | IC50=77 μM | 25750273 | ||
| U87 | Apoptosis Assay | 0–200 µM | 24 h | enhances CQ induced apoptosis | 25681668 | |
| U251MG | Apoptosis Assay | 100 μM | 48 h | PBS | induces apoptosis | 25680464 |
| U87MG | Apoptosis Assay | 100 μM | 48 h | PBS | induces apoptosis | 25680464 |
| U87 | Growth Inhibition Assay | 50-350 μM | 48 h | inhibits cell growth slightly | 25554223 | |
| U118 | Growth Inhibition Assay | 50-350 μM | 48 h | inhibits cell growth slightly | 25554223 | |
| U87 | Function Assay | 250/350 μM | 48 h | enhances TMX-induced p-PKC-pan decrease | 25554223 | |
| U118 | Function Assay | 250/350 μM | 48 h | enhances TMX-induced p-PKC-pan decrease | 25554223 | |
| U87 | Growth Inhibition Assay | 250/350 μM | 48 h | increases the percentage of cells in S and G2/M cotreated with TMX | 25554223 | |
| A375 | Growth Inhibition Assay | 48 h | IC50=265 μM | 25524552 | ||
| A2058 | Growth Inhibition Assay | 48 h | IC50=12 μM | 25524552 | ||
| M238 | Growth Inhibition Assay | 48 h | IC50=40 μM | 25524552 | ||
| M249 | Growth Inhibition Assay | 48 h | IC50=254 μM | 25524552 | ||
| M21 | Growth Inhibition Assay | 48 h | IC50=221 μM | 25524552 | ||
| U251 | Cytotoxity Assay | 20 μM | 48 h | reduceds the percentages of colonies formed | 25434381 | |
| LN229 | Cytotoxity Assay | 20 μM | 48 h | reduceds the percentages of colonies formed | 25434381 | |
| U373MG-LUC | Growth Inhibition Assay | 72 h | IC50>600 μM | 25431953 | ||
| U87 | Growth Inhibition Assay | 25-200 μM | 48 h | inhibits cell growth in a dose-dependent manner | 25400745 | |
| U251 | Growth Inhibition Assay | 25-200 μM | 48 h | inhibits cell growth in a dose-dependent manner | 25400745 | |
| U251 | Growth Inhibition Assay | 100-400 μM | 72/96 h | the anti-proliferative effect can be enhanced by gossypol enhanced | 25375271 | |
| U373 | Growth Inhibition Assay | 100-400 μM | 72/96 h | the anti-proliferative effect can be enhanced by gossypol enhanced | 25375271 | |
| U343 | Growth Inhibition Assay | 100-400 μM | 72/96 h | the anti-proliferative effect can be enhanced by gossypol enhanced | 25375271 | |
| U87MG-luc2 | Growth Inhibition Assay | 100-400 μM | 72/96 h | the anti-proliferative effect can be enhanced by gossypol enhanced | 25375271 | |
| U87 | Function Assay | 200 μM | 48 h | increases BRCC3 mRNA expression | 25337721 | |
| U251 | Function Assay | 200 μM | 48 h | increases BRCC3 mRNA expression | 25337721 | |
| A172 | Function Assay | 200 μM | 48 h | increases BRCC3 mRNA expression | 25337721 | |
| U251 | Function Assay | 200 μM | 48 h | increases the expression of BRCA1, BRCA2, RAD51 and FANCD2 | 25337721 | |
| A172 | Function Assay | 200 μM | 48 h | increases the expression of BRCA1, BRCA2, RAD51 and FANCD2 | 25337721 | |
| U87 | Function Assay | 200 μM | 24/72/120 h | increases γH2AX foci formation time-dependently | 25337721 | |
| U251 | Function Assay | 200 μM | 24/72/120 h | increases γH2AX foci formation time-dependently | 25337721 | |
| A172 | Function Assay | 200 μM | 24/72/120 h | increases γH2AX foci formation time-dependently | 25337721 | |
| SNB19V | Growth Inhibition Assay | 7 d | DMSO | GI50=35.7±12 μM | 25277441 | |
| SNB19M | Growth Inhibition Assay | 7 d | DMSO | GI50=469.9±88 μM | 25277441 | |
| SNB19VR | Growth Inhibition Assay | 7 d | DMSO | GI50=280.2±18 μM | 25277441 | |
| U373V | Growth Inhibition Assay | 7 d | DMSO | GI50=68.0±32 μM | 25277441 | |
| U373M | Growth Inhibition Assay | 7 d | DMSO | GI50=368.7±86 μM | 25277441 | |
| U373VR | Growth Inhibition Assay | 7 d | DMSO | GI50=288.8±33 μM | 25277441 | |
| U87MG | Growth Inhibition Assay | 7 d | DMSO | GI50=38.3±20 μM | 25277441 | |
| HCT116 | Growth Inhibition Assay | 7 d | DMSO | GI50=579.9±32 μM | 25277441 | |
| DLD1 | Growth Inhibition Assay | 7 d | DMSO | GI50=501.4±93 μM | 25277441 | |
| MRC5 | Growth Inhibition Assay | 7 d | DMSO | GI50=449.4±8 μM | 25277441 | |
| SNB19V | Function Assay | 100 μM TMZ | 0-72 h | increases γH2AX expression between 16 and 72 h | 25277441 | |
| T98G | Growth Inhibition Assay | 5/10/15 μM | 24 h | induces cell death dose-dependently after concomitant-temozolomide with NPe6-PDT | 25262961 | |
| U251 | Growth Inhibition Assay | 5/10/15 μM | 24 h | induces cell death dose-dependently after concomitant-temozolomide with NPe6-PDT | 25262961 | |
| T98G | Function Assay | 15 μM | 24 h | increases DNA-fragmentation in NPe6-PDT treated glioma cells | 25262961 | |
| U251 | Function Assay | 15 μM | 24 h | increases DNA-fragmentation in NPe6-PDT treated glioma cells | 25262961 | |
| U-87 MG | Growth Inhibition Assay | 72 h | IC50=0.93 mM | 25245332 | ||
| U-118 MG | Growth Inhibition Assay | 72 h | IC50=1.05 mM | 25245332 | ||
| U87 | Growth Inhibition Assay | 24 h | IC50=260.34 μM | 25173233 | ||
| U87 GSLCs | Growth Inhibition Assay | 24 h | IC50=766.11 μM | 25173233 | ||
| U87MG | Growth Inhibition Assay | 72 h | IC50=15.625 μM | 25050915 | ||
| U251 | Growth Inhibition Assay | 100-400 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| U87 | Growth Inhibition Assay | 100-400 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| MDA-MB-231-br | Growth Inhibition Assay | 0-10 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| HCC-1937 | Growth Inhibition Assay | 0-300 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| MDA-MB-231 | Growth Inhibition Assay | 0-40 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| MDA-MB-468 | Growth Inhibition Assay | 0-500 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| T47D | Growth Inhibition Assay | 0-100 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| MCF7 | Growth Inhibition Assay | 0-1000 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| Hs683 | Growth Inhibition Assay | 0-1000 μM | 96 h | IC50=128.9 μM | 24495907 | |
| U87 | Growth Inhibition Assay | 0-1000 μM | 96 h | IC50=18.45 μM | 24495907 | |
| LNZ308 | Growth Inhibition Assay | 0-1000 μM | 96 h | IC50=326.7 μM | 24495907 | |
| U87 | Apoptosis Assay | 100 μM | 48 h | DMSO | increases the caspase-3/7 activity | 24481586 |
| U251 | Apoptosis Assay | 100 μM | 48 h | DMSO | increases the caspase-3/7 activity | 24481586 |
| U251 | Growth Inhibition Assay | 24 h | IC50=86.29 ± 1.58 μM | 24326954 | ||
| U251 | Growth Inhibition Assay | 48 h | IC50=75.34 ± 1.02 μM | 24326954 | ||
| U251 | Growth Inhibition Assay | 72 h | IC50=72.42 ± 1.45 μM | 24326954 | ||
| U251 | Growth Inhibition Assay | 96 h | IC50=69.82 ± 3.04 μM | 24326954 | ||
| T98G | Growth Inhibition Assay | 0-750 μM | 72/96 h | inhibits cell viability in a dose dependent manner | 24324080 | |
| U251-MG | Growth Inhibition Assay | 0-800 μM | 72 h | inhibits cell viability in a dose dependent manner | 24093630 | |
| D54-MG | Growth Inhibition Assay | 0-800 μM | 72 h | inhibits cell viability in a dose dependent manner | 24093630 | |
| SHG-44 | Growth Inhibition Assay | 10-200 μM | 96 h | IC50=9.73 ± 2.12 μM | 24065569 | |
| U373 | Growth Inhibition Assay | 10-200 μM | 96 h | IC50=10.13 ± 1.02 μM | 24065569 | |
| HT-29 | Function Assay | 500 μM | 24/48 h | enhances the levels of γ-H2AX | 24038068 | |
| PC-3 | Growth Inhibition Assay | 0-25 μM | 48 h | inhibits cell growth which can be potentiated by lycopene | 23746934 | |
| PC-3 | Apoptosis Assay | 25 μM | 48 h | induces apoptosis which can be potentiated by lycopene | 23746934 | |
| T98G | Growth Inhibition Assay | 50-400 μM | 144 h | inhibits cell viability in a dose dependent manner | 23715499 | |
| U87-MG | Growth Inhibition Assay | 100 µM | 72 h | inhibits cell growth which can be enhanced by GTB | 23696788 | |
| U251-MG | Growth Inhibition Assay | 100 µM | 72 h | inhibits cell growth which can be enhanced by GTB | 23696788 | |
| LNT-229 | Growth Inhibition Assay | 3-100 μM | 24 h | inhibits clonogenic survival in a dose-dependent manner | 23667632 | |
| T98G | Growth Inhibition Assay | 10-700 μM | 24 h | inhibits clonogenic survival in a dose-dependent manner | 23667632 | |
| U87 | Function Assay | 100 µM | 3 h | elevates the levels of pChk1 and pChk2 | 23667469 | |
| HCT116 | Function Assay | 100 µM | 3 h | induces the Chk1 Phosphorylation | 23667469 | |
| HCT3-6 | Function Assay | 100 µM | 3 h | induces the Chk1 Phosphorylation | 23667469 | |
| U-87 | Growth Inhibition Assay | 0-40 μM | 12 d | inhibits cell growth in a dose-dependent manner | 23645729 | |
| U-87 | Apoptosis Assay | 0-40 μM | 3/6 d | induces apoptosis in both dose- and time-dependent manner | 23645729 | |
| U-87 | Function Assay | 0-40 μM | 3/6 d | induces autophagy in both dose- and time-dependent manner | 23645729 | |
| GB-SCC010 | Growth Inhibition Assay | 4 d | IC50=226 μM | 23612755 | ||
| GB-SCC026 | Growth Inhibition Assay | 4 d | IC50=53.1 μM | 23612755 | ||
| GB-SCC028 | Growth Inhibition Assay | 4 d | IC50=167 μM | 23612755 | ||
| U87 | Growth Inhibition Assay | 4 d | IC50=45.2 μM | 23612755 | ||
| U87 stem cell | Growth Inhibition Assay | 4 d | IC50=66.7 μM | 23612755 | ||
| TLX5 lymphoma | Cytotoxicity assay | IC50 = 5 μM | 7739008 | |||
| GM892 A | Cytotoxicity assay | IC50 = 7.6 μM | 7739008 | |||
| TLX5 lymphoma | Cytotoxicity assay | IC50 = 5 μM | 12459014 | |||
| HCT116 | Cytotoxicity assay | 4 days | IC50 = 4.34 μM | 19800803 | ||
| SNB75 | Antiproliferative assay | 10 uM | 24 hrs | Antiproliferative activity against human SNB75 cells at 10 uM after 24 hrs by SRB assay | 22268526 | |
| C6 | Antiproliferative assay | 100 uM | 48 hrs | Antiproliferative activity against rat C6 cells at 100 uM after 48 hrs by neutral red incorporation assay | 22268526 | |
| SF295 | Antiproliferative assay | 10 uM | 24 hrs | Antiproliferative activity against human SF295 cells at 10 uM after 24 hrs by SRB assay | 22268526 | |
| U87 | Antiproliferative assay | 5 days | IC50 = 49 μM | 22608389 | ||
| U138MG | Cytotoxicity assay | 48 hrs | IC50 = 26 μM | 23069682 | ||
| C6 | Cytotoxicity assay | 48 hrs | IC50 = 34 μM | 23069682 | ||
| A2780 | Antitumor assay | 5 days | IC50 = 8.5 μM | 23895620 | ||
| A2058 | Antitumor assay | 5 days | IC50 = 35.5 μM | 23895620 | ||
| SNB19 | Antitumor assay | 5 days | IC50 = 37 μM | 23895620 | ||
| M8 | Apoptosis assay | 50 to 100 uM | 48 hrs | Induction of apoptosis in human M8 cells assessed as apoptotic/necrotic cells at 50 to 100 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis | 24125877 | |
| SK-MEL-30 | Apoptosis assay | 100 uM | 48 hrs | Induction of apoptosis in human SK-MEL-30 cells assessed as apoptotic/necrotic cells at 100 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis relative to control | 24125877 | |
| SK-MEL-30 | Apoptosis assay | 50 uM | 48 hrs | Induction of apoptosis in human SK-MEL-30 cells assessed as apoptotic/necrotic cells at 50 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis relative to control | 24125877 | |
| MNT1 | Apoptosis assay | 100 uM | 48 hrs | Induction of apoptosis in human MNT1 cells assessed as apoptotic/necrotic cells at 100 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis relative to control | 24125877 | |
| MNT1 | Apoptosis assay | 50 uM | 48 hrs | Induction of apoptosis in human MNT1 cells assessed as apoptotic/necrotic cells at 50 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis relative to control | 24125877 | |
| A2780 | Cytotoxicity assay | 5 days | Cytotoxicity against human A2780 cells after 5 days by MTT assay | 24900418 | ||
| A2780/CP70 | Cytotoxicity assay | 5 days | Cytotoxicity against MMR-deficient human A2780/CP70 cells after 5 days by MTT assay | 24900418 | ||
| GBM 047T | Antitumor assay | 20 uM | 1 to 2 weeks | Tumoricidal effect in patient derived GBM 047T cells assessed as reduction in neurosphere formation at 20 uM after 1 to 2 weeks by 3D tumor clonogenic assay | 26355532 | |
| GBM 464T | Antitumor assay | 20 uM | 1 to 2 weeks | Tumoricidal effect in patient derived GBM 464T cells assessed as reduction in neurosphere formation at 20 uM after 1 to 2 weeks by 3D tumor clonogenic assay | 26355532 | |
| U87MG | Function assay | 3 hrs | Induction of DNA alkylation in human U87MG cells assessed as increase in N7-MedG formation after 3 hrs by LC-MS/MS analysis | 27614414 | ||
| U87MG | Antitumor assay | Antitumor activity against human U87MG cells orthotopically xenografted in Harlan nude mouse brain assessed as induction of slow tumor growth at 50 umol/kg, iv administered once daily for 5 days | 27614414 | |||
| U87MG | Antitumor assay | Antitumor activity against human U87MG cells orthotopically xenografted in Harlan nude mouse brain assessed as increase in mouse survival at 50 umol/kg, iv administered once daily for 5 days | 27614414 | |||
| MDCK | Cytotoxicity assay | 24 hrs | Cytotoxicity against MDCK cells expressing carbonic anhydrase 9 assessed as reduction in cell viability incubated for 24 hrs measured after 7 days under normoxic condition by methylene blue staining based clonogenic survival assay | 27823879 | ||
| MDCK | Cytotoxicity assay | 24 hrs | Cytotoxicity against MDCK cells expressing carbonic anhydrase 9 assessed as reduction in cell viability incubated for 24 hrs measured after 7 days under hypoxic condition by methylene blue staining based clonogenic survival assay | 27823879 | ||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| C6 | Cytotoxicity assay | 4 days | EC50 = 16.5 μM | ChEMBL | ||
| U87 | Cytotoxicity assay | 72 hrs | IC50 = 19.38 μM | ChEMBL | ||
| SNB19 | Growth inhibition assay | 7 days | GI50 = 35.7 μM | ChEMBL | ||
| SNB19 | Growth inhibition assay | 7 days | GI50 = 45.6 μM | ChEMBL | ||
| U373 | Function assay | 100 uM | 72 hrs | Induction of double stranded DNA break in empty vector transfected human U373 cells assessed as increase in gamma-H2AX level at 100 uM after 72 hrs by flow cytometry | ChEMBL | |
| Glioma | Antitumor assay | Antitumor activity against Homo sapiens (human) Glioma cells xenografted in transgenic mouse assessed as mouse survival | ChEMBL | |||
| Klicken Sie hier, um weitere experimentelle Daten zu Zelllinien anzuzeigen | ||||||
Chemische Informationen, Lagerung & Stabilität
| Molekulargewicht | 194.15 | Formel | C6H6N6O2 |
Lagerung (Ab dem Eingangsdatum) | 3 years-20°C (in the dark)powder |
|---|---|---|---|---|---|
| CAS-Nr. | 85622-93-1 | SDF herunterladen | Lagerung von Stammlösungen |
|
|
| Synonyme | NSC 362856,CCRG 81045,Methazolastone | Smiles | CN1C(=O)N2C=NC(=C2N=N1)C(=O)N | ||
Löslichkeit
|
In vitro |
DMSO
: 39 mg/mL
(200.87 mM)
Mit 50°C Wasserbad erwärmt;
Ultraschallbehandelt;
Water : 10 mg/mL (超声加热五分钟) Ethanol : Insoluble |
Molaritätsrechner
|
In vivo |
|||||
In-vivo-Formulierungsrechner (Klare Lösung)
Schritt 1: Geben Sie die untenstehenden Informationen ein (Empfohlen: Ein zusätzliches Tier zur Berücksichtigung von Verlusten während des Experiments)
Schritt 2: Geben Sie die In-vivo-Formulierung ein (Dies ist nur der Rechner, keine Formulierung. Bitte kontaktieren Sie uns zuerst, wenn es im Abschnitt "Löslichkeit" keine In-vivo-Formulierung gibt.)
Berechnungsergebnisse:
Arbeitskonzentration: mg/ml;
Methode zur Herstellung der DMSO-Stammlösung: mg Wirkstoff vorgelöst in μL DMSO ( Konzentration der Stammlösung mg/mL, Bitte kontaktieren Sie uns zuerst, wenn die Konzentration die DMSO-Löslichkeit der Wirkstoffcharge überschreitet. )
Methode zur Herstellung der In-vivo-Formulierung: Nehmen Sie μL DMSO Stammlösung, dann hinzufügenμL PEG300, mischen und klären, dann hinzufügenμL Tween 80, mischen und klären, dann hinzufügen μL ddH2O, mischen und klären.
Methode zur Herstellung der In-vivo-Formulierung: Nehmen Sie μL DMSO Stammlösung, dann hinzufügen μL Maisöl, mischen und klären.
Hinweis: 1. Bitte stellen Sie sicher, dass die Flüssigkeit klar ist, bevor Sie das nächste Lösungsmittel hinzufügen.
2. Achten Sie darauf, das/die Lösungsmittel der Reihe nach hinzuzufügen. Sie müssen sicherstellen, dass die bei der vorherigen Zugabe erhaltene Lösung eine klare Lösung ist, bevor Sie mit der Zugabe des nächsten Lösungsmittels fortfahren. Physikalische Methoden wie Vortex, Ultraschall oder ein heißes Wasserbad können zur Unterstützung des Lösens verwendet werden.
Wirkmechanismus
| Merkmale |
Methazolastone is a second-generation alkylating agent.
|
|---|---|
| Targets/IC50/Ki |
DNA replication
(L-1210, L-1210/BCNU cells) |
| In vitro |
Temozolomide (TMZ) verursacht die Bildung von alkali-labilen DNA-Stellen, die in ähnlichen Mengen vorhanden sind und mit ähnlicher Rate in L-1210- und L-1210/BCNU-Zelllinien repariert werden. In L-1210, aber nicht in L-1210/BCNU, induziert diese Verbindung einen Arrest der Zellen in den SL-G2-M-Phasen. Ihre Empfindlichkeit sowohl von chemo-sensitiven als auch von resistenten Zellen (D54-R und U87-R) wird unter Hyperoxie signifikant erhöht. Sowohl sie als auch Hyperoxie sind mit einer erhöhten Phosphorylierung von ERK p44/42 MAPK (Erk1/2) verbunden, jedoch in geringerem Maße in D54-R-Zellen, was darauf hindeutet, dass die Erk1/2-Aktivität an der Regulation des durch Hyperoxie und TMZ vermittelten Zelltods beteiligt sein könnte. Hyperoxie verstärkt ihre Toxizität in GBM-Zellen durch Induktion von Apoptosis, möglicherweise über MAPK-bezogene Signalwege. Sie induziert in Monozyten die DNA Damage Response Pathways ATM-Chk2 und ATR-Chk1, was zu einer p53-Aktivierung führt. Chronische Exposition gegenüber dieser Verbindung führt zu erworbener TMZ-Resistenz und erhöht die miR-21-Expression. Die Behandlung damit löst endoplasmatischen Retikulum (ER)-Stress mit erhöhter Expression von GADD153- und GRP78-Proteinen aus und vermindert das Pro-Caspase-12-Protein. Es induziert Autophagy durch mitochondrialen Schädigung- und ER-Stress-abhängige Mechanismen, um Gliomzellen zu schützen. |
| In vivo |
Nach einer täglichen i.p.-Dosis von 40 mg/kg über 5 aufeinanderfolgende Tage (Tage 1-5 nach Tumortransplantation) erhöht TMZ (Temozolomide) die Lebensdauer bei L-1210 um 86 % und bei L-1210/BCNU um 22 %. Bei L-1210/BCNU wird nach 100 μM oder 200 μM Behandlung kein Effekt beobachtet; nur 400 μM dieser Verbindung führten zu einer Akkumulation von Zellen in der prämotischen Phase, jedoch viel weniger als bei L-1210. Bei L-1210/BCNU beträgt die maximale Akkumulation von Zellen in SL-G2-M nach 48-72 Stunden ungefähr 30 % im Vergleich zu 23 % in unbehandelten Zellen. Zellen akkumulierten auch in SL-G2-M, wenn L-1210-Leukämie-tragende Mäuse i.v. damit behandelt wurden (40 mg/kg). Bei L-1210/BCNU-Zellen von Mäusen, denen die gleiche Medikamentendosis verabreicht wurde, wurde kein solcher Effekt beobachtet. |
Literatur |
|
Anwendungen
| Methoden | Biomarker | Bilder | PMID |
|---|---|---|---|
| Western blot | pERK / ERK / p-p38 / p38 DR5 / c-FLIP / Survivin / XIAP |
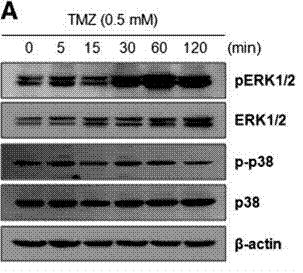
|
24436439 |
| Growth inhibition assay | Cell viability |
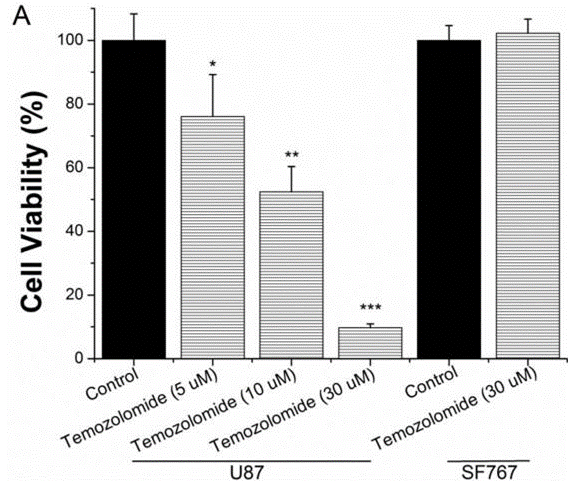
|
25751281 |
| Immunofluorescence | Phalloidin / Phospho-H2A.X cleaved caspase-3 |
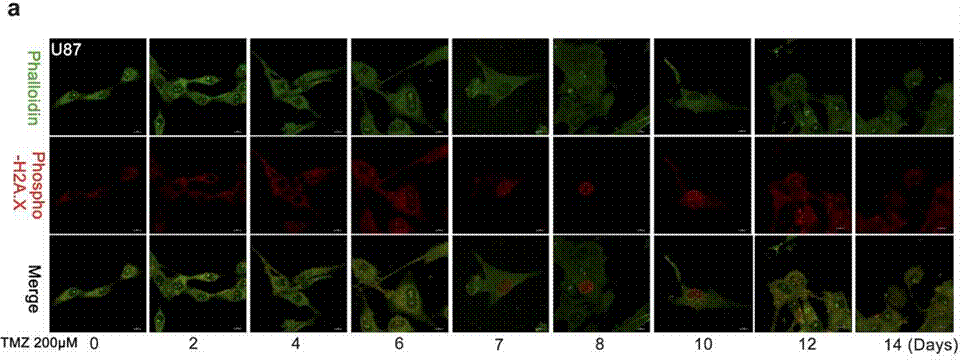
|
27375225 |
Klinische Studieninformationen
(Daten von https://clinicaltrials.gov, aktualisiert am 2024-05-22)
| NCT-Nummer | Rekrutierung | Erkrankungen | Sponsor/Kooperationspartner | Startdatum | Phasen |
|---|---|---|---|---|---|
| NCT05128734 | Not yet recruiting | Breast Cancer Triple Negative |
AHS Cancer Control Alberta |
July 1 2024 | Phase 2 |
| NCT06161974 | Not yet recruiting | High Grade Glioma|Astrocytoma|Astrocytoma Grade III|Astrocytoma Grade IV|Diffuse Intrinsic Pontine Glioma|WHO Grade III Glioma|WHO Grade IV Glioma|Metastatic Brain Tumor|Diffuse Midline Glioma H3 K27M-Mutant|Thalamus Tumor|Spinal Tumor|IDH1 Mutation|IDH1 R132|IDH1 R132C|IDH1 R132H|IDH1 R132S|IDH1 R132G|IDH1 R132L|Oligodendroglioma |
Rigel Pharmaceuticals|Nationwide Children''s Hospital |
June 2024 | Phase 2 |
| NCT04967690 | Not yet recruiting | Newly Diagnosed Glioblastoma |
Double Bond Pharmaceutical AB |
January 2024 | Phase 1 |
| NCT05698524 | Recruiting | Recurrent High Grade Glioma|Anaplastic Astrocytoma|Anaplastic Oligodendroglioma|Glioblastoma|Gliosarcoma |
University of Nebraska|Xynomic Pharmaceuticals Inc. |
June 26 2023 | Phase 1 |
| NCT04945148 | Not yet recruiting | Glioblastoma IDH-wildtype |
Hopital Foch|National Cancer Institute France |
May 2023 | Phase 2 |
Technischer Support
Tel: +1-832-582-8158 Ext:3
Wenn Sie weitere Fragen haben, hinterlassen Sie bitte eine Nachricht.
