Inhibitor of apoptosis proteins (IAPs) constitute a family of evolutionarily conserved regulatory molecules that play pivotal roles in modulating cell survival and death pathways, making them attractive targets for therapeutic intervention in various diseases, particularly cancer. IAP inhibitors, as small-molecule or biological agents designed to disrupt the anti-apoptotic functions of IAPs, have emerged as a promising class of compounds in preclinical and clinical research. Over the past two decades, extensive scientific efforts have been devoted to elucidating the molecular mechanisms underlying IAP-mediated apoptosis suppression, optimizing the structure and efficacy of IAP inhibitors, and exploring their therapeutic potential in combination with other treatment modalities. This article focuses on the scientific landscape of IAP inhibitors, with an emphasis on their interaction with inhibitor of apoptosis proteins, the mechanism of action, and recent advances in preclinical and clinical research.
IAP Inhibitoren (IAP Inhibitors)
Weitere Apoptosis Inhibitoren
| Kat.-Nr. | Produktname | Informationen | Publikationen | Validierung |
|---|---|---|---|---|
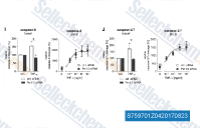
| S7597 | BV6 | BV-6 ist ein SMAC-Mimetikum, ein dualer cIAP- und XIAP-Inhibitor. |
|
|
| S7089 | SM-164 | SM-164 ist ein potenter, nicht-peptidischer, zellgängiger Antagonist von XIAP, der sowohl die BIR2- als auch die BIR3-Domänen mit einer IC50 von 1,39 nM anspricht. Diese Verbindung induziert Apoptose und Tumorrückbildung. |
|
|
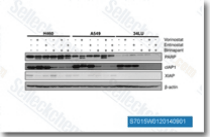
| S7015 | Birinapant (TL32711) | Birinapant ist ein SMAC-mimetischer Antagonist, hauptsächlich von cIAP1 mit einem Kd von <1 nM in einem zellfreien Assay, weniger potent gegenüber XIAP. Diese Verbindung hilft, die Apoptose in latent HIV-1-infizierten Zellen zu induzieren. Phase 2. |
|
|
| S7009 | LCL161 | LCL-161, ein niedermolekulares second mitochondrial activator of caspase (SMAC)-Mimetikum, bindet potent an mehrere IAPs (d.h. XIAP, c-IAP) und hemmt diese. |
|

|
| S2754 | Xevinapant (AT406) | Xevinapant (AT406, ARRY-334543, Debio1143, SM-406) ist ein potenter Smac-Mimetikum und ein Antagonist von IAP (Inhibitor des Apoptose-Proteins über E3 Ubiquitinligase), der an XIAP-BIR3, cIAP1-BIR3 und cIAP2-BIR3 mit Ki von 66,4 nM, 1,9 nM und 5,1 nM bindet, 50- bis 100-fach höhere Affinitäten als das Smac AVPI-Peptid. Phase 1. |
|

|
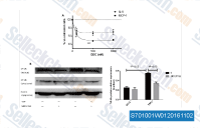
| S7010 | GDC-0152 | GDC-0152 ist ein potenter Antagonist von XIAP-BIR3, ML-IAP-BIR3, cIAP1-BIR3 und cIAP2-BIR3 mit Ki von 28 nM, 14 nM, 17 nM bzw. 43 nM in zellfreien Assays; geringere Affinität wurde zu cIAP1-BIR2 und cIAP2-BIR2 gezeigt. Phase 1. |
|
|
| S7362 | AZD5582 | AZD5582, ein neuartiger niedermolekularer IAP-Inhibitor, bindet potent an die BIR3-Domänen von cIAP1, cIAP2 und XIAP mit IC50-Werten von 15, 21 und 15 |
|
|
| S8681 | Tolinapant (ASTX660) | Tolinapant (ASTX660) ist ein potenter, nicht-peptidomimetischer Antagonist von cIAP1/2 und XIAP, der die Wechselwirkungen zwischen einem SMAC-abgeleiteten Peptid und den BIR3-Domänen von XIAP (BIR3-XIAP) und cIAP1 (BIR3-cIAP1) mit IC50-Werten von weniger als 40 bzw. 12 nmol/L hemmt. |
|
|
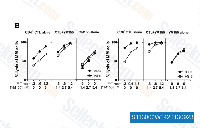
| S1130 | Sepantronium Bromide (YM155) | Sepantronium Bromide (YM155) ist ein potenter Survivin-Suppressor, der die Survivin-Promoteraktivität mit einer IC50 von 0,54 nM in HeLa-SURP-luc- und CHO-SV40-luc-Zellen hemmt. Es hemmt die SV40-Promoteraktivität nicht signifikant, aber es wird beobachtet, dass es die Interaktion von Survivin mit XIAP leicht hemmt. Diese Verbindung reguliert Survivin und XIAP herunter, moduliert die Autophagy und induziert Autophagy-abhängige DNA-Schäden in Brustkrebszellen. Phase 2. |
|
|
| S2271 | Berberine chloride | Berberine chloride ist ein quaternäres Ammoniumsalz aus der Gruppe der Isochinolin-Alkaloide. Diese Verbindung aktiviert Caspase 3 und Caspase 8, die Spaltung von Poly-ADP-Ribose-Polymerase (PARP) und die Freisetzung von Cytochrom c. Es verringert die Expression von c-IAP1, Bcl-2 und Bcl-XL. Diese Chemikalie induziert Apoptosis mit anhaltender Phosphorylierung von JNK und p38 MAPK sowie die Erzeugung von ROS. Es ist ein dualer Topoisomerase I- und II-Inhibitor. Es ist auch ein potenzieller Autophagy-Modulator. |
|
|
Inhibitor of Apoptosis Proteins: Key Regulators of Apoptotic Pathways
Apoptosis, or programmed cell death, is a tightly regulated physiological process essential for maintaining tissue homeostasis, eliminating damaged or abnormal cells, and preventing tumorigenesis. Dysregulation of apoptosis is a hallmark of cancer, where cancer cells evade cell death through various mechanisms, including overexpression of anti-apoptotic proteins such as IAPs. The IAP family comprises several members, including X-linked inhibitor of apoptosis protein (XIAP), cellular inhibitor of apoptosis protein 1 (cIAP1), cellular inhibitor of apoptosis protein 2 (cIAP2), and survivin, each with distinct structural and functional characteristics.
A defining feature of IAPs is the presence of one or more baculoviral IAP repeat (BIR) domains, which are crucial for their anti-apoptotic activity. These BIR domains mediate protein-protein interactions with caspases, the key effector molecules of apoptosis, thereby inhibiting caspase activation and subsequent cell death. For instance, XIAP, the most well-characterized member of the IAP family, binds to caspases-3, -7, and -9 through its BIR2 and BIR3 domains, directly blocking their proteolytic activity. In addition to inhibiting caspases, IAPs also participate in other signaling pathways, such as the nuclear factor-κB (NF-κB) pathway, which further contributes to cell survival, proliferation, and inflammation. The multifaceted roles of IAPs in promoting cell survival make them ideal targets for the development of anti-cancer agents, leading to the design and synthesis of a wide range of IAP inhibitors.
Mechanism of Action of IAP Inhibitors
The core mechanism of action of IAP inhibitors revolves around disrupting the interaction between IAPs and caspases, thereby restoring the apoptotic capacity of cancer cells. To achieve this, IAP inhibitors are designed to mimic the natural antagonists of IAPs, such as Smac/DIABLO (second mitochondria-derived activator of caspases/direct IAP-binding protein with low pI) and HtrA2/Omi. Smac/DIABLO is a mitochondrial protein that is released into the cytoplasm upon apoptotic stimulation, where it binds to the BIR domains of IAPs with high affinity, displacing caspases and allowing their activation.
Smac Mimetics: The Major Class of IAP Inhibitors
The majority of IAP inhibitors developed to date are Smac mimetics, small molecules that mimic the N-terminal AVPI (Ala-Val-Pro-Ile) tetrapeptide motif of Smac/DIABLO, which is critical for binding to the BIR domains of IAPs. By mimicking this motif, Smac mimetics compete with caspases for binding to IAPs, leading to the release and activation of caspases, ultimately triggering apoptotic cell death. For example, compounds such as LCL161, birinapant, and AZD5582 are well-studied Smac mimetics that have shown potent activity against a variety of cancer cell lines in preclinical studies. These compounds bind to XIAP, cIAP1, and cIAP2, inhibiting their anti-apoptotic functions and inducing apoptosis in cancer cells that overexpress these IAPs.
Additional Mechanisms of IAP Inhibitor Action
Beyond displacing caspases, IAP inhibitors exhibit other mechanisms of action that contribute to their anti-cancer efficacy. One such mechanism is the induction of cIAP1 and cIAP2 degradation. Upon binding to Smac mimetics, cIAP1 and cIAP2 undergo auto-ubiquitination and subsequent proteasomal degradation. The degradation of cIAPs leads to the activation of the non-canonical NF-κB pathway, which can promote apoptosis in certain cancer cell types by upregulating the expression of pro-apoptotic genes. Additionally, IAP inhibitors can sensitize cancer cells to other anti-cancer treatments, such as chemotherapy, radiation therapy, and immunotherapy. For example, combining Smac mimetics with chemotherapeutic agents like cisplatin or paclitaxel has been shown to enhance apoptotic cell death in cancer cells that are resistant to chemotherapy alone, likely by overcoming the anti-apoptotic barrier imposed by IAPs.
Preclinical and Clinical Research Advances in IAP Inhibitors
Preclinical studies have demonstrated the broad anti-tumor activity of IAP inhibitors across various cancer types, including pancreatic cancer, ovarian cancer, non-small cell lung cancer, and melanoma. In these studies, IAP inhibitors have been shown to induce apoptosis in cancer cells, inhibit tumor growth in xenograft mouse models, and enhance the efficacy of other anti-cancer therapies. For instance, birinapant has been shown to inhibit tumor growth in xenograft models of ovarian cancer and melanoma, and to sensitize these tumors to radiation therapy.
Clinical Trials of IAP Inhibitors
Based on promising preclinical results, several IAP inhibitors have advanced to clinical trials to evaluate their safety, tolerability, and efficacy in cancer patients. Early-phase clinical trials (Phase I and II) have been conducted for compounds such as LCL161, birinapant, AZD5582, and AT-406. These trials have shown that IAP inhibitors are generally well-tolerated, with manageable side effects such as fatigue, nausea, vomiting, and diarrhea. However, the single-agent efficacy of IAP inhibitors in clinical trials has been modest, with only a small number of patients achieving partial responses or stable disease.
Combination Therapy Strategies in Clinical Research
Given the modest single-agent activity, current clinical research is focused on exploring combination therapy strategies involving IAP inhibitors and other anti-cancer agents. For example, combinations of IAP inhibitors with chemotherapy (e.g., paclitaxel, docetaxel), immunotherapy (e.g., PD-1/PD-L1 inhibitors), and targeted therapy (e.g., B RAF inhibitors) are being evaluated in clinical trials. The rationale behind these combinations is to exploit the ability of IAP inhibitors to sensitize cancer cells to other treatments, thereby enhancing overall therapeutic efficacy. Preliminary results from some of these combination trials have been encouraging. For instance, a Phase II trial combining birinapant with paclitaxel in patients with advanced ovarian cancer showed a higher objective response rate compared to paclitaxel alone. Similarly, combinations of IAP inhibitors with PD-1/PD-L1 inhibitors have shown promise in preclinical studies and are currently being evaluated in clinical trials for various cancer types.
Challenges and Future Directions in IAP Inhibitor Research
Despite significant progress in the field of IAP inhibitor research, several challenges remain to be addressed. One major challenge is the development of resistance to IAP inhibitors. Preclinical studies have shown that cancer cells can develop resistance to Smac mimetics through various mechanisms, including upregulation of alternative anti-apoptotic proteins (e.g., Bcl-2, Bcl-xL), downregulation of caspases, and mutations in IAPs that reduce their binding affinity for Smac mimetics. Understanding the mechanisms of resistance is crucial for the development of strategies to overcome it, such as combining IAP inhibitors with inhibitors of alternative anti-apoptotic pathways.
Another challenge is the lack of predictive biomarkers to identify patients who are most likely to benefit from IAP inhibitor therapy. Currently, there are no validated biomarkers that can reliably predict the response to IAP inhibitors in cancer patients. Identifying such biomarkers would enable personalized medicine approaches, allowing clinicians to select patients who are likely to respond to treatment and avoid unnecessary treatment in non-responders. Future research efforts should focus on identifying and validating predictive biomarkers, such as the expression levels of specific IAPs, caspases, or other components of the apoptotic pathway.
In conclusion, IAP inhibitors represent a promising class of anti-cancer agents that target the anti-apoptotic functions of inhibitor of apoptosis proteins. The mechanism of action of these inhibitors, primarily through mimicking Smac/DIABLO to displace caspases and induce IAP degradation, has been well-characterized in preclinical studies. While single-agent efficacy in clinical trials has been modest, combination therapy strategies involving IAP inhibitors and other anti-cancer agents have shown encouraging results. Addressing the challenges of resistance and identifying predictive biomarkers will be critical for the successful clinical translation of IAP inhibitors. With continued scientific research and clinical development, IAP inhibitors have the potential to become an important component of personalized cancer therapy in the future.
